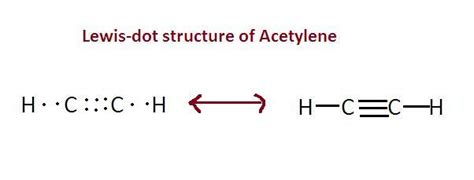
Acetylene, also known as ethyne, is a hydrocarbon compound with the chemical formula C2H2. It is the simplest alkyne, consisting of two carbon atoms bonded by a triple bond, with each carbon atom also bonded to a hydrogen atom. Understanding the Lewis structure of acetylene is crucial for grasping its chemical properties and reactivity. The Lewis structure, developed by Gilbert N. Lewis, is a graphical representation of the distribution of electrons in a molecule, which helps in predicting the shape, polarity, and reactivity of the molecule.
To draw the Lewis structure of acetylene, one must follow a series of steps. First, determine the total number of valence electrons in the molecule. Carbon has 4 valence electrons, and hydrogen has 1. Since there are two carbon atoms and two hydrogen atoms in acetylene, the total number of valence electrons is (2*4) + (2*1) = 10. Next, the atoms are connected by single bonds, which account for 4 electrons (2 bonds * 2 electrons per bond), leaving 6 electrons to be distributed. Each carbon atom then forms a triple bond, which consists of one sigma bond and two pi bonds, utilizing 4 of the remaining electrons. The last 2 electrons are placed on the sigma bond between the carbons, fulfilling the octet rule for each carbon atom and indicating a triple bond between the two carbon atoms.
Key Points
- Acetylene's chemical formula is C2H2, indicating it is the simplest alkyne.
- The molecule consists of a carbon-carbon triple bond and two carbon-hydrogen single bonds.
- Drawing the Lewis structure involves determining the total valence electrons (10 for C2H2), connecting atoms with single bonds, and then distributing the remaining electrons to form a triple bond between the carbon atoms.
- The triple bond in acetylene consists of one sigma bond and two pi bonds, explaining its linear geometry and high reactivity.
- Understanding the Lewis structure of acetylene is essential for predicting its chemical properties and reactivity in various reactions.
Chemical Properties and Reactivity
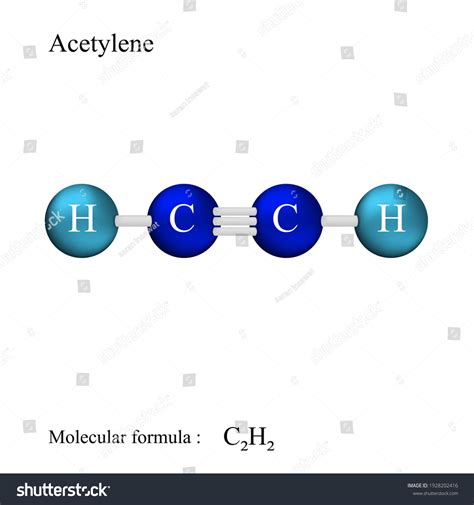
The unique triple bond in acetylene’s Lewis structure significantly influences its chemical properties and reactivity. This bond, with its combination of a sigma and two pi bonds, is highly reactive. Acetylene can undergo addition reactions, where molecules add across the triple bond, converting it into a single or double bond. For example, acetylene reacts with hydrogen in the presence of a catalyst to form ethane (C2H6), and it reacts with chlorine to form dichloroethene (C2H2Cl2). The reactivity of acetylene is also exploited in its use as a building block in organic synthesis and in the production of plastics and other chemicals.
Synthesis and Industrial Applications
Acetylene is synthesized on an industrial scale through the hydrolysis of calcium carbide (CaC2), which reacts with water to produce acetylene and calcium hydroxide. This process is represented by the equation CaC2 + 2H2O → C2H2 + Ca(OH)2. The gas is then purified and can be used in various applications. Acetylene is used in welding and cutting metals, as it can produce a high-temperature flame when burned with oxygen. It is also a precursor to many organic compounds, including vinyl chloride (the precursor to PVC), and is used in the synthesis of certain pharmaceuticals and agrochemicals.
| Compound | Chemical Reaction | Products |
|---|---|---|
| Acetylene + Hydrogen | C2H2 + 2H2 → C2H6 | Ethane |
| Acetylene + Chlorine | C2H2 + Cl2 → C2H2Cl2 | Dichloroethene |
| Calcium Carbide + Water | CaC2 + 2H2O → C2H2 + Ca(OH)2 | Acetylene, Calcium Hydroxide |
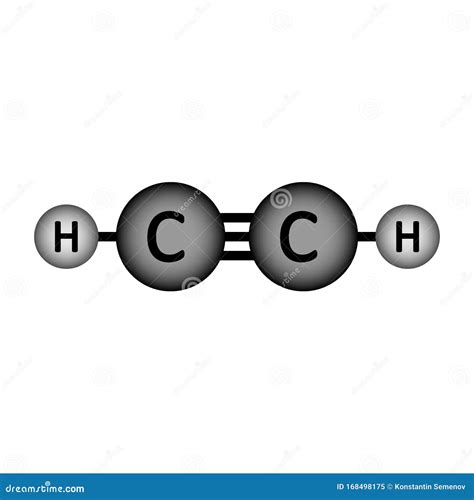
Conclusion and Future Perspectives

In conclusion, the Lewis structure of acetylene provides fundamental insights into its molecular geometry, chemical properties, and reactivity. Understanding these aspects is crucial for exploiting acetylene’s potential in organic synthesis, materials science, and industrial processes. As research continues to advance, the development of new catalysts and methodologies for manipulating acetylene and other alkynes will likely expand their applications in chemistry and materials science, further highlighting the significance of foundational knowledge about molecular structures and properties.
What is the simplest way to draw the Lewis structure of acetylene?
+To draw the Lewis structure of acetylene, start by determining the total number of valence electrons (10 for C2H2), then connect the atoms with single bonds, and finally distribute the remaining electrons to form a triple bond between the carbon atoms.
What are the main industrial applications of acetylene?
+Acetylene is used in welding and cutting metals due to its ability to produce a high-temperature flame when burned with oxygen. It is also a precursor to many organic compounds, including vinyl chloride (the precursor to PVC), and is used in the synthesis of certain pharmaceuticals and agrochemicals.
How does the triple bond in acetylene influence its reactivity?
+The triple bond in acetylene makes it highly reactive, as it can undergo addition reactions where molecules add across the triple bond, converting it into a single or double bond. This reactivity is crucial for its applications in organic synthesis and industrial chemistry.