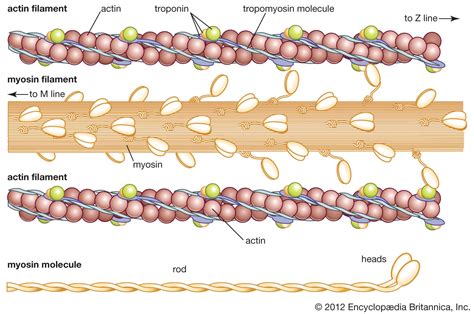
Actin and myosin are two of the most crucial proteins found in muscles, responsible for the contraction and relaxation mechanisms that enable movement and maintain posture. The complex interplay between these proteins is fundamental to understanding how muscles function, from the molecular level to the physiological effects on the human body. Actin, a globular protein, and myosin, a motor protein, work in tandem to facilitate muscle contraction through a process known as the sliding filament theory. This intricate process involves the myosin heads binding to actin filaments and using ATP hydrolysis to produce a power stroke, which moves the actin filaments along the myosin filaments, thereby contracting the muscle fiber.
The study of actin and myosin has a rich history, dating back to the early 20th century when the first detailed descriptions of muscle structure and function began to emerge. Over the years, advances in microscopy, biochemistry, and molecular biology have significantly expanded our understanding of these proteins and their role in muscle physiology. Today, research continues to uncover the nuances of actin-myosin interactions, including the regulatory mechanisms that fine-tune muscle contraction in response to neural stimuli. This knowledge has not only deepened our appreciation for the complexity of muscle function but also informed the development of therapeutic strategies for muscle-related disorders.
Key Points
- The actin-myosin interaction is the basis of muscle contraction, enabling movement and maintaining posture.
- The sliding filament theory explains how actin and myosin filaments slide past each other to contract muscle fibers.
- ATP hydrolysis provides the energy required for the myosin power stroke, which drives muscle contraction.
- Regulatory proteins, such as tropomyosin and troponin, play critical roles in modulating the actin-myosin interaction in response to calcium ions.
- Understanding the molecular mechanisms of actin and myosin is crucial for developing treatments for muscle diseases and disorders.
Molecular Structure and Function of Actin and Myosin
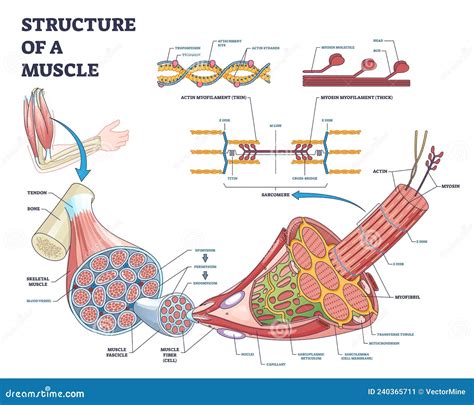
Actin filaments, also known as F-actin, are composed of two strands of actin monomers (G-actin) twisted together. Myosin, on the other hand, is a hexameric protein consisting of two heavy chains and four light chains. The heavy chains have globular heads that bind to actin and a long tail that interacts with other myosin molecules to form thick filaments. The binding of myosin heads to actin filaments and the subsequent power stroke are essential for muscle contraction. The process is highly regulated, with the presence of calcium ions playing a pivotal role in initiating contraction by binding to troponin, which shifts the position of tropomyosin, exposing the myosin binding sites on actin.
Regulation of Actin-Myosin Interaction
The regulation of the actin-myosin interaction is a complex process involving several proteins. Tropomyosin and troponin are key regulatory proteins found on the actin filaments. In the absence of calcium ions, tropomyosin blocks the myosin binding sites on actin, preventing contraction. When a muscle is stimulated to contract by a nerve impulse, calcium ions are released into the sarcoplasm, bind to troponin, and cause a conformational change that moves tropomyosin away from the myosin binding sites, allowing myosin to bind to actin and initiate contraction. This regulatory mechanism ensures that muscle contraction is tightly controlled and occurs only when necessary, preventing unnecessary energy expenditure and potential damage to muscle fibers.
| Protein | Function |
|---|---|
| Actin | Forms thin filaments that interact with myosin to produce contraction |
| Myosin | Forms thick filaments with heads that bind to actin and produce the power stroke for contraction |
| Tropomyosin | Regulatory protein that blocks myosin binding sites on actin in the absence of calcium ions |
| Troponin | Regulatory protein that binds calcium ions and initiates contraction by shifting tropomyosin |
Clinical Relevance of Actin and Myosin
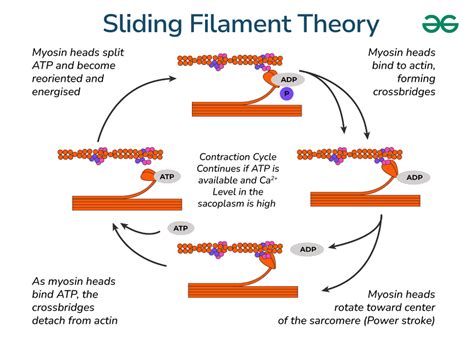
Disorders affecting the actin and myosin proteins or their regulatory mechanisms can lead to significant muscle dysfunction. Muscular dystrophies, for example, are a group of genetic disorders characterized by progressive muscle weakness and degeneration. In Duchenne muscular dystrophy, mutations in the dystrophin gene lead to the absence of the dystrophin protein, which is critical for maintaining the integrity of muscle fibers during contraction. The lack of dystrophin disrupts the normal function of the actin cytoskeleton and leads to muscle cell damage and death. Similarly, hypertrophic cardiomyopathy can result from mutations in genes encoding myosin heavy chains or other sarcomeric proteins, leading to abnormal muscle contraction and potential heart failure.
Therapeutic Strategies
Research into the molecular mechanisms of actin and myosin has opened avenues for the development of targeted therapies for muscle diseases. Gene therapy, aiming to correct the genetic defects underlying muscular dystrophies, is a promising approach. Additionally, drugs that modulate the actin-myosin interaction or enhance muscle strength and function are under investigation. For instance, drugs that increase the availability of utrophin, a protein that can partially compensate for the lack of dystrophin, are being explored as potential treatments for Duchenne muscular dystrophy. The understanding of actin and myosin biology also informs the development of novel therapeutic strategies, such as exon skipping and CRISPR-Cas9 gene editing, which aim to correct the genetic mutations causing these diseases.
What is the primary mechanism by which actin and myosin interact to produce muscle contraction?
+The primary mechanism involves the myosin heads binding to actin filaments and using ATP hydrolysis to produce a power stroke, moving the actin filaments along the myosin filaments and thereby contracting the muscle fiber.
How are the actin-myosin interactions regulated in muscle fibers?
+The interactions are regulated by the presence of calcium ions, which bind to troponin and cause tropomyosin to shift away from the myosin binding sites on actin, allowing myosin to bind and initiate contraction.
What are some potential therapeutic strategies for disorders affecting actin and myosin?
+Potential therapeutic strategies include gene therapy to correct genetic defects, drugs that modulate the actin-myosin interaction, and novel approaches like exon skipping and CRISPR-Cas9 gene editing.
In conclusion, the actin and myosin proteins and their interactions are fundamental to muscle function, and their dysregulation can lead to significant muscle disorders. The ongoing research into the molecular mechanisms of these proteins and their regulatory mechanisms not only deepens our understanding of muscle biology but also holds promise for the development of effective therapeutic strategies for muscle diseases.