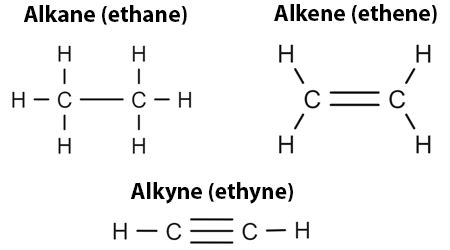
The realm of organic chemistry is replete with fascinating compounds, and among them, alkanes, alkenes, and alkynes are three fundamental types of hydrocarbons. These compounds are the building blocks of organic chemistry, and understanding their differences is crucial for advancing in this field. Alkanes, alkenes, and alkynes are classified based on their molecular structure, specifically the type of carbon-carbon bonds they contain. In this article, we will delve into the differences between these three types of hydrocarbons, exploring their structures, properties, and reactions.
Key Points
- Alkanes are saturated hydrocarbons with single bonds between carbon atoms.
- Alkenes are unsaturated hydrocarbons with at least one double bond between carbon atoms.
- Alkynes are unsaturated hydrocarbons with at least one triple bond between carbon atoms.
- The type of carbon-carbon bond in these compounds influences their physical and chemical properties.
- Understanding the differences between alkanes, alkenes, and alkynes is essential for predicting their reactivity and applications.
Alkanes: Structure and Properties
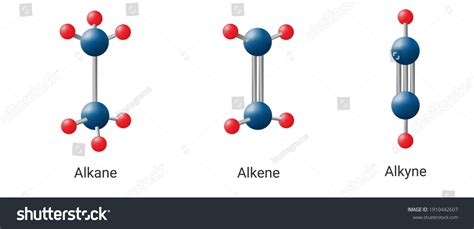
Alkanes are saturated hydrocarbons, meaning they only contain single bonds between carbon atoms. Their general molecular formula is CnH2n+2, where n represents the number of carbon atoms. The simplest alkane is methane (CH4), followed by ethane (C2H6), propane (C3H8), and butane (C4H10). Alkanes are typically non-polar, resulting in low melting and boiling points. They are also relatively unreactive due to their stable molecular structure.
Alkenes: Introduction to Unsaturated Hydrocarbons
Alkenes, on the other hand, are unsaturated hydrocarbons that contain at least one double bond between carbon atoms. Their general molecular formula is CnH2n, where n represents the number of carbon atoms. The simplest alkene is ethene (C2H4), also known as ethylene. Alkenes are more reactive than alkanes due to the presence of double bonds, which are electron-rich and susceptible to attack by electrophiles. This reactivity makes alkenes useful in various chemical reactions, such as addition reactions.
Alkynes: The Triple Bond Difference
Alkynes are also unsaturated hydrocarbons but contain at least one triple bond between carbon atoms. Their general molecular formula is CnH2n-2, where n represents the number of carbon atoms. The simplest alkyne is ethyne (C2H2), also known as acetylene. Alkynes are even more reactive than alkenes due to the increased electron density around the triple bond. This reactivity is utilized in various chemical reactions, including addition reactions and polymerization processes.
| Type of Hydrocarbon | Molecular Formula | Example |
|---|---|---|
| Alkane | CnH2n+2 | Methane (CH4) |
| Alkene | CnH2n | Ethene (C2H4) |
| Alkyne | CnH2n-2 | Ethyne (C2H2) |
Comparative Analysis of Alkanes, Alkenes, and Alkynes
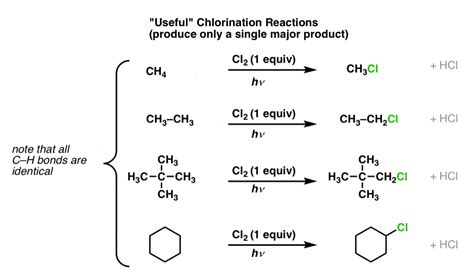
A comparative analysis of these hydrocarbons reveals that their differences in molecular structure lead to distinct physical and chemical properties. Alkanes, with their single bonds, are generally less reactive and have lower melting and boiling points compared to alkenes and alkynes. Alkenes and alkynes, with their double and triple bonds, respectively, exhibit higher reactivity due to the increased electron density around these bonds. This reactivity is a critical factor in determining their applications and the types of chemical reactions they undergo.
Reactions of Alkanes, Alkenes, and Alkynes
The type of carbon-carbon bond in these hydrocarbons significantly influences their reactivity. Alkanes primarily undergo substitution reactions, where a functional group replaces a hydrogen atom. Alkenes and alkynes, due to their unsaturated nature, are more prone to addition reactions, where molecules add across the double or triple bond. Understanding these reaction patterns is essential for predicting the outcomes of chemical reactions involving these compounds.
What are the main differences between alkanes, alkenes, and alkynes?
+The primary difference lies in the type of carbon-carbon bonds they contain: alkanes have single bonds, alkenes have at least one double bond, and alkynes have at least one triple bond.
How does the type of carbon-carbon bond affect the reactivity of these hydrocarbons?
+The presence of double or triple bonds in alkenes and alkynes, respectively, increases their reactivity compared to alkanes. This is due to the higher electron density around these bonds, making them more susceptible to attack by electrophiles.
What are some common applications of alkanes, alkenes, and alkynes?
+Alkanes are used as fuels and in the production of plastics. Alkenes are crucial in the manufacture of polymers, such as polyethylene and polypropylene, and in the production of pharmaceuticals. Alkynes are used in the synthesis of complex molecules and in the production of certain plastics and fibers.
In conclusion, the differences between alkanes, alkenes, and alkynes are fundamental to understanding their properties, reactivity, and applications. By recognizing the implications of their molecular structures, chemists can predict and manipulate their behavior, leading to the development of new materials, drugs, and technologies. As research in organic chemistry continues to evolve, the distinctions between these hydrocarbons will remain a cornerstone of understanding and innovation in the field.
Meta Description: Explore the fundamental differences between alkanes, alkenes, and alkynes, including their structures, properties, and reactions, to understand their roles in organic chemistry and their various applications.