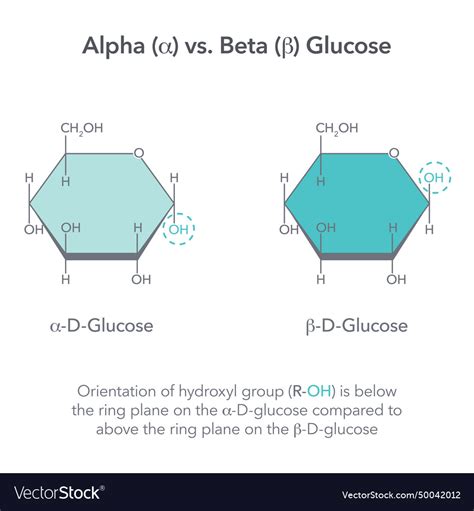
The distinction between alpha and beta glucose is a fundamental concept in carbohydrate chemistry, particularly in the context of glycosidic bonds and the structure of polysaccharides. Glucose, a simple sugar (monosaccharide), exists in two primary anomeric forms: alpha (α) and beta (β), which differ in the orientation of the hydroxyl group (-OH) attached to the anomeric carbon (the carbon derived from the aldehyde or ketone group of the sugar). This difference in orientation significantly affects the chemical and biological properties of glucose molecules and their polymers.
Understanding the alpha vs beta glucose difference is crucial in various fields, including biochemistry, nutrition, and pharmaceuticals. The human body utilizes glucose as a primary source of energy, and the distinction between alpha and beta configurations influences how glucose is metabolized and utilized by cells. Furthermore, the alpha and beta forms of glucose are integral components of different polysaccharides, such as starch (primarily alpha-1,4 linkages) and cellulose (primarily beta-1,4 linkages), which have vastly different functions and digestibilities in the human diet.
Key Points
- The alpha and beta forms of glucose differ in the orientation of the hydroxyl group attached to the anomeric carbon.
- This difference affects the chemical and biological properties of glucose molecules and their polymers.
- Alpha and beta glucose configurations are crucial in the structure of polysaccharides like starch and cellulose.
- The distinction between alpha and beta glucose is significant in human metabolism and nutrition.
- Enzymes and chemical reactions can convert between alpha and beta glucose forms.
Naturally Occurring Forms of Glucose
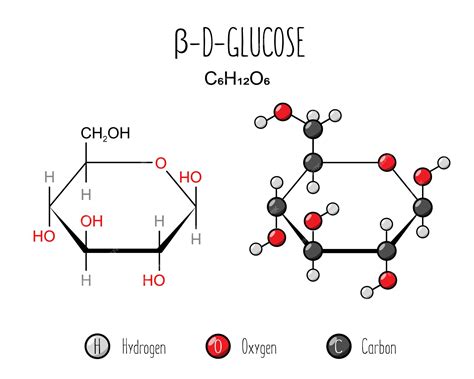
In nature, glucose is predominantly found in the form of polysaccharides, such as starch in plants and glycogen in animals. Starch, for instance, is composed of amylose and amylopectin, both of which contain alpha-1,4 glycosidic bonds. These bonds are responsible for the helical structure of amylose and the branched structure of amylopectin. The alpha configuration allows for more efficient packing of the glucose molecules, contributing to the compact structure of starch granules in plants.
Chemical and Biological Implications
The chemical and biological properties of alpha and beta glucose are distinct due to the differences in their three-dimensional structures. The orientation of the hydroxyl group on the anomeric carbon influences the reactivity of the molecule, affecting how easily it can form bonds with other molecules. For example, enzymes like amylase, which breaks down starch into simpler sugars, are specific to alpha-1,4 glycosidic bonds, illustrating the biological significance of the alpha configuration in glucose metabolism.
| Type of Glucose | Description | Biological Significance |
|---|---|---|
| Alpha Glucose | D-GLucose with the hydroxyl group below the plane of the ring | Primary component of starch and glycogen; easily metabolized by humans |
| Beta Glucose | D-GLucose with the hydroxyl group above the plane of the ring | Primary component of cellulose; not easily digestible by humans |
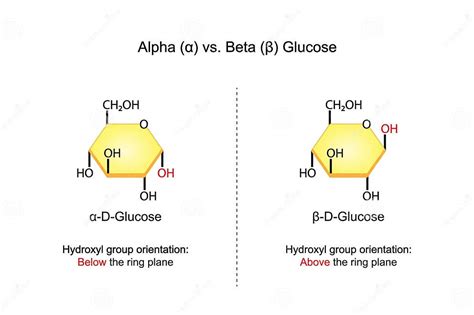
Conversion Between Alpha and Beta Glucose
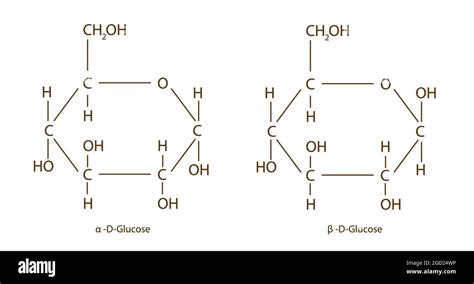
The conversion between alpha and beta glucose forms is a process known as mutarotation. This process occurs spontaneously in solution, where the ring form of glucose opens to form an aldehyde, which then recloses to either the alpha or beta form. Enzymes can also catalyze this conversion, with some enzymes preferring one form over the other. The ability to convert between alpha and beta glucose is essential in various biological pathways, including glycolysis, where glucose is broken down to produce energy.
Industrial and Pharmaceutical Applications
The difference between alpha and beta glucose has significant implications for industrial and pharmaceutical applications. For instance, the production of biofuels from cellulose requires the breakdown of beta-1,4 glycosidic bonds, a process that is more challenging than the breakdown of alpha-1,4 bonds in starch. Additionally, the design of drugs that target specific glycosidic bonds or the development of nutritional supplements that are easily digestible by humans must consider the alpha vs beta glucose difference.
What is the primary difference between alpha and beta glucose?
+The primary difference lies in the orientation of the hydroxyl group attached to the anomeric carbon, with alpha glucose having the hydroxyl group below the plane of the ring and beta glucose having it above.
Why is the distinction between alpha and beta glucose important in human nutrition?
+This distinction is crucial because it affects the digestibility and metabolism of glucose-containing polysaccharides. Alpha-linked polysaccharides like starch are easily broken down by human enzymes, while beta-linked polysaccharides like cellulose are not.
Can enzymes convert between alpha and beta glucose forms?
+Yes, certain enzymes can catalyze the conversion between alpha and beta glucose forms. This process is important in various biological pathways and industrial applications.
In conclusion, the difference between alpha and beta glucose is a critical aspect of carbohydrate chemistry, with significant implications for biology, nutrition, and industry. Understanding these differences is essential for advancing our knowledge of biochemical processes, developing new technologies, and improving human health. As research continues to unravel the complexities of glucose metabolism and the roles of alpha and beta glucose in various biological pathways, the importance of this distinction will only continue to grow.