The Advanced Placement (AP) Chemistry course is designed to provide students with a comprehensive understanding of chemical principles and their applications. To excel in this course, it is essential to grasp the fundamental concepts and units that underlie the subject. In this article, we will delve into the essential units of AP Chemistry, exploring the key topics, concepts, and skills that students need to master to succeed in the course.
Key Points
- Atomic structure and periodic trends are foundational concepts in AP Chemistry
- Chemical bonding, including Lewis structures and molecular orbital theory, is crucial for understanding molecular properties
- Thermodynamics, kinetics, and equilibrium are essential topics in AP Chemistry, with a focus on calculations and problem-solving
- Acid-base chemistry, including strong and weak acids and bases, is a critical area of study
- Lab techniques and safety protocols are vital components of the AP Chemistry course
Atomic Structure and Periodic Trends

The atomic structure and periodic trends unit introduces students to the fundamental principles of chemistry, including the structure of atoms, electron configuration, and periodic trends. This unit lays the foundation for understanding chemical bonding, thermodynamics, and other key concepts in AP Chemistry. Students should be able to explain the principles of atomic structure, including the proton, neutron, and electron, and describe the periodic trends of elements, such as atomic radius, electronegativity, and ionization energy.
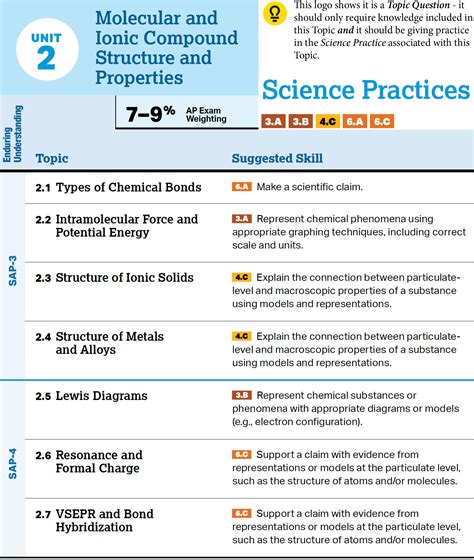
Chemical Bonding
Chemical bonding is a critical unit in AP Chemistry, as it explains how atoms interact with each other to form molecules. Students should be able to describe the different types of chemical bonds, including ionic, covalent, and metallic bonds, and explain the principles of Lewis structures and molecular orbital theory. This unit also introduces students to the concept of polarity and intermolecular forces, which are essential for understanding the physical and chemical properties of molecules.
| Chemical Bond Type | Characteristics |
|---|---|
| Ionic Bond | Transfer of electrons between atoms, resulting in the formation of ions |
| Covalent Bond | Sharing of electrons between atoms, resulting in the formation of a molecule |
| Metallic Bond | Delocalization of electrons among a lattice of metal atoms |

Thermodynamics, Kinetics, and Equilibrium
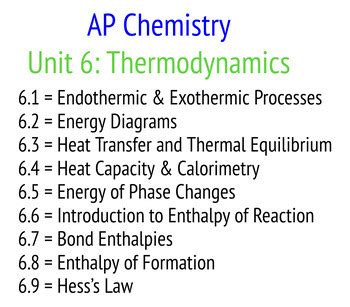
Thermodynamics, kinetics, and equilibrium are essential topics in AP Chemistry, as they explain the energy changes that occur during chemical reactions and the rates at which these reactions occur. Students should be able to calculate the energy changes associated with chemical reactions, including enthalpy, entropy, and Gibbs free energy, and describe the principles of kinetics, including reaction rates and activation energy. This unit also introduces students to the concept of equilibrium, including the equilibrium constant and Le Chatelier’s principle.
Acid-Base Chemistry
Acid-base chemistry is a critical area of study in AP Chemistry, as it explains the behavior of acids and bases in aqueous solutions. Students should be able to describe the principles of acid-base chemistry, including the Arrhenius, Bronsted-Lowry, and Lewis definitions of acids and bases, and explain the concept of pH and pOH. This unit also introduces students to the concept of strong and weak acids and bases, including their dissociation constants and conjugate acid-base pairs.
Lab Techniques and Safety Protocols
Lab techniques and safety protocols are vital components of the AP Chemistry course, as they provide students with hands-on experience with chemical experiments and introduce them to the principles of laboratory safety. Students should be able to describe the principles of laboratory safety, including the use of personal protective equipment, chemical handling, and waste disposal, and demonstrate proficiency in laboratory techniques, including titration, chromatography, and spectroscopy.
What are the key concepts in AP Chemistry?
+The key concepts in AP Chemistry include atomic structure and periodic trends, chemical bonding, thermodynamics, kinetics, and equilibrium, acid-base chemistry, and lab techniques and safety protocols.
How do I prepare for the AP Chemistry exam?
+To prepare for the AP Chemistry exam, students should review the course material, practice problems, and lab techniques, and take practice exams to assess their knowledge and skills.
What are the benefits of taking AP Chemistry?
+The benefits of taking AP Chemistry include advanced placement in college chemistry courses, improved critical thinking and problem-solving skills, and enhanced preparation for careers in science, technology, engineering, and mathematics (STEM).
In conclusion, the essential units of AP Chemistry provide students with a comprehensive understanding of chemical principles and their applications. By mastering the key concepts and skills in these units, students can succeed in the course and prepare themselves for advanced studies in chemistry and related fields.