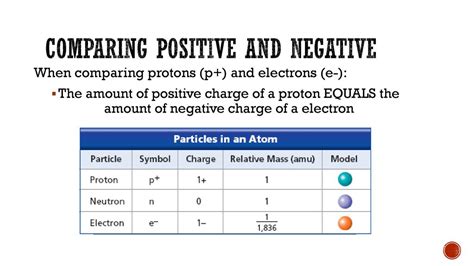
The concept of electron charge is fundamental to understanding the behavior of matter at the atomic and subatomic level. The electron, a negatively charged particle, plays a crucial role in the structure and properties of atoms, influencing chemical reactions, physical interactions, and the overall characteristics of elements. Here, we delve into five key facts about electron charge, exploring its significance, measurement, and implications in various scientific contexts.
Key Points
- The electron charge is a fundamental constant of nature, denoted as e and approximately equal to -1.602 x 10^-19 Coulombs.
- The quantization of electron charge is a cornerstone of quantum mechanics, implying that electrons can only occupy specific energy levels or shells around the nucleus.
- The electron charge affects the chemical properties of elements, with the number of electrons in an atom determining its reactivity and ability to form compounds.
- Electron charge is crucial in understanding electrical conductivity, with materials classified as conductors, semiconductors, or insulators based on their ability to facilitate the flow of electrons.
- Recent advances in physics have led to a deeper understanding of electron charge in relation to quantum computing and the development of new materials with unique electrical properties.
Understanding Electron Charge: A Fundamental Constant
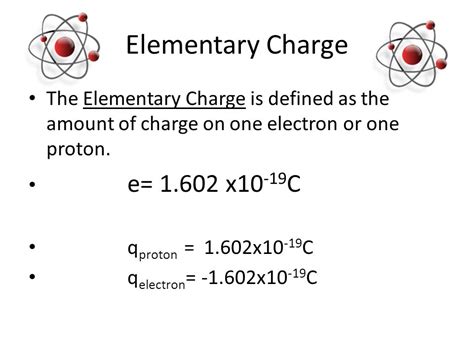
The charge of an electron is a fundamental constant in physics, denoted by the symbol e and measured in Coulombs ©. This constant is approximately equal to -1.602 x 10^-19 C. The negative charge of the electron is what distinguishes it from other subatomic particles, such as protons, which carry a positive charge. The precise measurement of electron charge has been the subject of extensive scientific research, with experiments continually refining our understanding of this constant and its implications for physical phenomena.
The Quantization of Electron Charge
A key aspect of electron charge is its quantization, a principle stating that electrons can only occupy specific energy levels or shells around the nucleus of an atom. This concept is central to quantum mechanics and explains the discrete nature of atomic spectra. The quantization of electron charge leads to the periodic table of elements, where atoms with similar numbers of electrons in their outermost shell exhibit similar chemical properties. This understanding has profound implications for chemistry, materials science, and our comprehension of the physical world.
| Element | Number of Electrons | Chemical Properties |
|---|---|---|
| Hydrogen | 1 | Highly reactive, forms compounds easily |
| Helium | 2 | Noble gas, unreactive |
| Oxygen | 8 | Highly reactive, essential for combustion |
Electron Charge and Chemical Properties
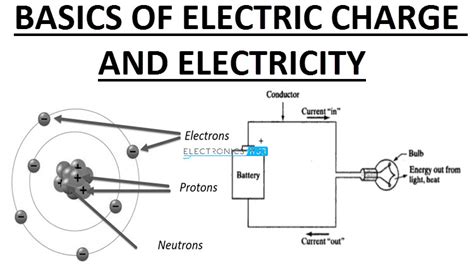
The number of electrons in an atom, particularly those in the outermost shell, determines its chemical properties and reactivity. Elements with a full outer shell are generally unreactive (noble gases), while those with partially filled outer shells are more reactive. This principle underlies the periodic table’s structure, where elements are arranged by their atomic number (number of protons) and exhibit periodic trends in their chemical behavior. The electron configuration, which describes the distribution of electrons in an atom, is essential for predicting chemical reactions and the formation of compounds.
Electrical Conductivity and Electron Charge
Electron charge is also pivotal in understanding electrical conductivity. Materials can be classified as conductors, semiconductors, or insulators based on their ability to facilitate the flow of electrons. Conductors, such as metals, have electrons that are relatively free to move, allowing for the efficient transfer of electrical charge. Semiconductors, used in electronic devices, have a intermediate level of conductivity that can be controlled through doping. Insulators, on the other hand, have tightly bound electrons that do not participate in electrical conduction. The manipulation of electron charge in these materials is crucial for the development of electronic devices and technologies.
As research continues to advance our understanding of electron charge and its implications, new areas of study are emerging. The field of quantum computing, for example, relies on the manipulation of electron spin and charge to create qubits, the fundamental units of quantum information. Additionally, the development of new materials with unique electrical properties, such as superconductors and nanomaterials, is driven by a deeper understanding of electron charge and its role in determining material properties.
What is the significance of electron charge in chemistry?
+The electron charge is crucial in chemistry as it determines the reactivity of elements and their ability to form compounds. The number of electrons in an atom's outermost shell influences its chemical properties, making electron charge a fundamental concept in understanding chemical reactions and the periodic table.
How does electron charge affect electrical conductivity?
+Electron charge affects electrical conductivity by determining the ability of materials to facilitate the flow of electrons. Materials with free electrons (conductors) allow for efficient electrical conduction, while those with tightly bound electrons (insulators) do not. The manipulation of electron charge is essential for controlling electrical conductivity and developing electronic devices.
What are the implications of electron charge for quantum computing?
+The manipulation of electron charge is critical for quantum computing, as it enables the creation of qubits. The control of electron spin and charge allows for the encoding and processing of quantum information, making electron charge a fundamental aspect of quantum computing technology.
In conclusion, electron charge is a fundamental concept that underlies many phenomena in physics and chemistry. Its significance extends from the atomic structure and chemical reactivity of elements to the electrical conductivity of materials and the development of quantum computing technologies. As science continues to unravel the mysteries of electron charge, new insights and innovations are emerging, promising to revolutionize our understanding of the physical world and the technologies that shape our lives.