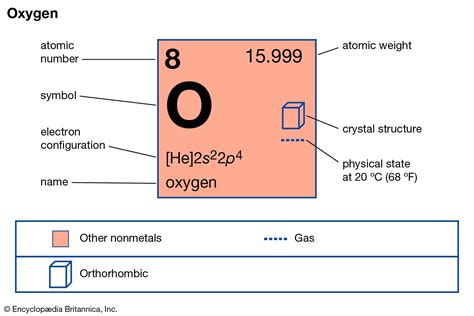
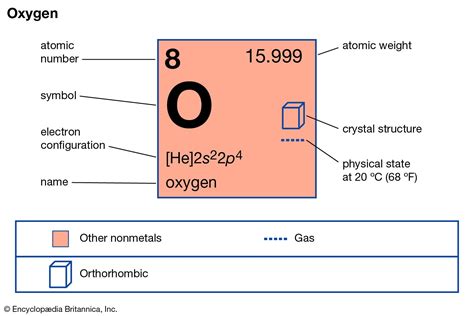
Oxygen, a fundamental element in the periodic table, has an atomic mass of approximately 15.9994 u (unified atomic mass units). This value is a weighted average of the masses of the naturally occurring isotopes of oxygen, including oxygen-16, oxygen-17, and oxygen-18. Understanding the atomic mass of oxygen is crucial in various fields, including chemistry, physics, and biology, as it plays a significant role in determining the properties and behavior of molecules and compounds. In this article, we will explore five ways the atomic mass of oxygen impacts our understanding of the world around us.
Atomic Mass and Isotopic Abundance

The atomic mass of oxygen is not a fixed value but rather a weighted average that takes into account the relative abundance of its isotopes. Oxygen-16 is the most abundant isotope, making up about 99.76% of natural oxygen, followed by oxygen-17 and oxygen-18, which contribute 0.0378% and 0.2049%, respectively. The precise measurement of these isotopes and their relative abundance is essential for calculating the atomic mass of oxygen. For instance, the atomic mass can be calculated using the formula: (0.9976 * 15.994915) + (0.000378 * 16.999132) + (0.002049 * 17.999161), which yields an average atomic mass close to 15.9994 u.
Chemical Reactions and Stoichiometry
In chemical reactions, the atomic mass of oxygen is critical for determining the stoichiometry of reactants and products. Stoichiometry is the quantitative relationship between reactants and products in chemical reactions. The atomic mass of oxygen helps in calculating the number of moles of oxygen required or produced in a reaction, which is essential for predicting the amounts of other reactants or products involved. For example, in the combustion of methane (CH4), the reaction equation is CH4 + 2O2 → CO2 + 2H2O. Knowing the atomic mass of oxygen (15.9994 u) allows us to calculate the molar mass of oxygen (31.9988 g/mol), which is crucial for determining the stoichiometric ratios in this reaction.
| Isotope | Natural Abundance (%) | Atomic Mass (u) |
|---|---|---|
| Oxygen-16 | 99.76 | 15.994915 |
| Oxygen-17 | 0.0378 | 16.999132 |
| Oxygen-18 | 0.2049 | 17.999161 |
Biological Importance of Oxygen
Oxygen is essential for life as we know it, particularly in the process of cellular respiration, where oxygen acts as the final electron acceptor in the electron transport chain, enabling the production of ATP (adenosine triphosphate), the energy currency of the cell. The atomic mass of oxygen, while not directly influencing its biological role, underpins our understanding of the biochemical processes that rely on oxygen. For instance, the molar mass of oxygen, derived from its atomic mass, is used in calculations related to the respiratory quotient, which is the ratio of the volume of carbon dioxide produced to the volume of oxygen consumed during respiration.
Applications in Materials Science
In materials science, the atomic mass of oxygen is significant in the study and development of oxide materials, which have a wide range of applications, from electronics and catalysis to energy storage and conversion. The precise control of oxygen stoichiometry in these materials can dramatically affect their properties. For example, in the development of solid oxide fuel cells (SOFCs), the atomic mass of oxygen is crucial for understanding the transport of oxygen ions through the electrolyte material, which is typically an oxide ceramic. The efficiency and performance of SOFCs depend on the ability to control and optimize this oxygen ion transport.
Key Points
- The atomic mass of oxygen is a weighted average of its naturally occurring isotopes, primarily oxygen-16, oxygen-17, and oxygen-18.
- Understanding the atomic mass of oxygen is crucial for stoichiometric calculations in chemical reactions and for determining the properties of molecules and compounds.
- Oxygen's biological importance, particularly in cellular respiration, is underpinned by its atomic mass, which is essential for calculations related to biochemical processes.
- In materials science, the atomic mass of oxygen plays a significant role in the development and understanding of oxide materials, including those used in electronics, catalysis, and energy applications.
- The precise measurement and calculation of oxygen's atomic mass contribute to advancements in geochemical, environmental, and biological sciences.
As we continue to explore the intricacies of the natural world and push the boundaries of technological innovation, the atomic mass of oxygen remains a foundational piece of knowledge. Its impact is felt across disciplines, from the simplest biochemical reactions to the most complex materials and technologies. The ongoing refinement of oxygen's atomic mass and our understanding of its isotopes will undoubtedly continue to illuminate new pathways for scientific inquiry and application.
What is the significance of oxygen's atomic mass in chemistry?
+Oxygen's atomic mass is crucial for calculating the molar masses of compounds containing oxygen, which is essential for stoichiometric calculations in chemical reactions.
How does the atomic mass of oxygen affect its biological role?
+While the atomic mass of oxygen does not directly influence its biological role, understanding its atomic mass underpins the calculations and processes related to oxygen's participation in biochemical reactions, such as cellular respiration.
What are some applications of oxygen's atomic mass in materials science?
+The atomic mass of oxygen is significant in the study and development of oxide materials, which have applications in electronics, catalysis, energy storage, and conversion. It affects the understanding and control of oxygen stoichiometry in these materials.
Meta Description: Explore the significance of oxygen’s atomic mass and its impact across chemistry, biology, and materials science, with a focus on its role in chemical reactions, cellular respiration, and the development of oxide materials.