The atomic number of potassium is a fundamental concept in chemistry, and understanding its significance is crucial for grasping the properties and behavior of this essential element. Potassium, denoted by the symbol K, is an alkali metal with an atomic number of 19. This means that a potassium atom has 19 protons in its atomic nucleus, which defines its position in the periodic table and determines its chemical properties.
The atomic number is a unique identifier for each element, and it plays a critical role in chemistry, physics, and materials science. In the case of potassium, its atomic number of 19 indicates that it has 19 protons and 19 electrons in a neutral atom. The number of protons in an atom's nucleus determines the element's identity and its position in the periodic table, while the number of electrons determines its chemical reactivity and properties.
Potassium is a soft, silvery-white metal that is highly reactive and readily loses one electron to form a positive ion, known as a cation. This reactivity is due to its low ionization energy, which is a result of its atomic number and the configuration of its electrons. The atomic number of 19 also influences potassium's chemical properties, such as its ability to form compounds with other elements and its role in biological systems.
Key Points
- The atomic number of potassium is 19, which defines its position in the periodic table and determines its chemical properties.
- Potassium has 19 protons and 19 electrons in a neutral atom, which influences its reactivity and properties.
- The atomic number of potassium is crucial for understanding its chemical behavior, including its ability to form compounds and its role in biological systems.
- Potassium is a highly reactive element, readily losing one electron to form a positive ion, due to its low ionization energy.
- The atomic number of 19 also influences potassium's physical properties, such as its melting and boiling points, and its reactivity with other elements.
Potassium’s Position in the Periodic Table
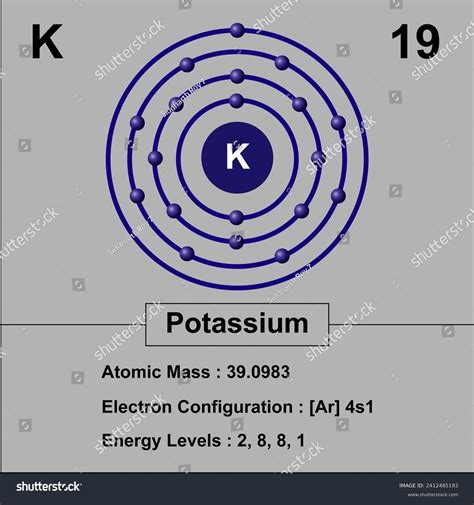
Potassium’s atomic number of 19 places it in the first column of the periodic table, along with other alkali metals such as lithium, sodium, and rubidium. This column is characterized by elements with a single electron in their outermost energy level, which makes them highly reactive and prone to losing that electron to form a positive ion. The periodic table is a powerful tool for understanding the relationships between elements and predicting their chemical properties, and potassium’s position in the table provides valuable insights into its behavior.
Chemical Properties of Potassium
Potassium’s chemical properties are significantly influenced by its atomic number of 19. Its low ionization energy makes it highly reactive, and it readily loses one electron to form a positive ion. This reactivity is also influenced by the configuration of its electrons, which is determined by the atomic number. Potassium’s chemical properties include its ability to form compounds with other elements, such as oxides, halides, and sulfides, and its role in biological systems, where it plays a crucial role in maintaining fluid balance and regulating nerve function.
| Property | Value |
|---|---|
| Atomic Number | 19 |
| Atomic Mass | 39.0983 u |
| Electron Configuration | [Ar] 4s1 |
| Ionization Energy | 418.8 kJ/mol |
| Electronegativity | 0.82 |
Applications of Potassium

Potassium’s unique properties make it an essential element in various applications. Its high reactivity and low ionization energy make it a valuable component in the production of potassium-based compounds, such as potassium hydroxide, potassium nitrate, and potassium sulfate. These compounds have numerous applications in industries such as agriculture, manufacturing, and pharmaceuticals. Additionally, potassium’s role in biological systems makes it an important element in maintaining human health, and its isotopes are used in medical imaging and research.
In conclusion, the atomic number of potassium is a fundamental concept that underlies its chemical properties and behavior. Understanding the significance of this number is essential for appreciating the unique characteristics of potassium and its role in various applications. By recognizing the relationships between potassium's atomic number, its position in the periodic table, and its chemical properties, researchers and scientists can better appreciate the complexities of this essential element.
What is the atomic number of potassium?
+The atomic number of potassium is 19, which defines its position in the periodic table and determines its chemical properties.
What are the chemical properties of potassium?
+Potassium’s chemical properties include its high reactivity, low ionization energy, and ability to form compounds with other elements. Its atomic number of 19 influences its chemical behavior and determines its position in the periodic table.
What are the applications of potassium?
+Potassium’s unique properties make it an essential element in various applications, including the production of potassium-based compounds, agriculture, manufacturing, pharmaceuticals, and medical imaging.



