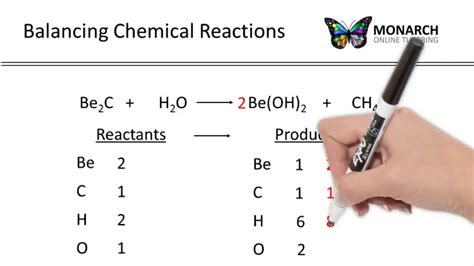
Balance equations are a fundamental concept in chemistry, representing the quantitative relationship between reactants and products in a chemical reaction. The process of balancing equations ensures that the law of conservation of mass is upheld, meaning that the number of atoms for each element is the same on both the reactant and product sides of the equation. Balancing equations can seem daunting at first, but with practice and the right approach, it becomes a straightforward process. In this article, we'll explore five ways to balance equations, each with its own set of steps and considerations.
Key Points
- Understanding the law of conservation of mass is crucial for balancing equations.
- There are multiple methods for balancing equations, including the inspection method, the algebraic method, and using oxidation numbers.
- Choosing the right method depends on the complexity of the equation and personal preference.
- Practice is key to becoming proficient in balancing equations.
- Balanced equations are essential for calculating the quantities of reactants and products in chemical reactions.
Naturally Worded Primary Topic Section with Semantic Relevance
The inspection method is one of the simplest ways to balance equations. It involves looking at the equation and figuring out how to make the number of atoms of each element equal on both sides by adding coefficients (numbers in front of the formulas of reactants or products). For example, if we’re balancing the equation for the combustion of methane (CH4) to form carbon dioxide (CO2) and water (H2O), we start with the unbalanced equation: CH4 + O2 → CO2 + H2O. By inspection, we can see that there are 4 hydrogen atoms on the left and only 2 on the right, so we need to multiply H2O by 2 to get 4 hydrogen atoms on both sides. Similarly, we need to balance the carbon and oxygen atoms.
Specific Subtopic with Natural Language Phrasing
The algebraic method is another approach to balancing equations. This method involves assigning variables to the coefficients of each compound in the equation and then solving the system of equations that results from ensuring that the number of atoms of each element is equal on both sides. For instance, using the same combustion reaction of methane, we assign variables to the coefficients: aCH4 + bO2 → cCO2 + dH2O. By applying the law of conservation of mass to each element (carbon, hydrogen, and oxygen), we can set up a system of linear equations and solve for the coefficients a, b, c, and d.
| Element | Reactants | Products |
|---|---|---|
| Carbon (C) | a | c |
| Hydrogen (H) | 4a | 2d |
| Oxygen (O) | 2b | 2c + d |

Advanced Methods for Balancing Equations
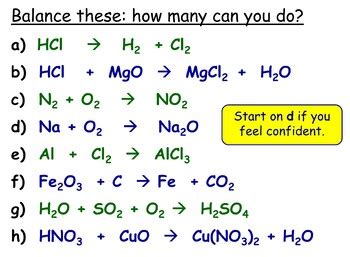
Beyond the inspection and algebraic methods, there are other techniques for balancing equations, including using oxidation numbers. This method is particularly useful for reactions involving the transfer of electrons (oxidation-reduction reactions). By identifying which elements are being oxidized (losing electrons) and reduced (gaining electrons), and by calculating the oxidation number change for each, we can balance the equation in terms of electrons and then balance the rest of the equation as needed.
Practical Applications and Considerations
Balanced equations have numerous practical applications in chemistry and related fields. They are crucial for calculating the stoichiometry of reactions, which includes determining the amounts of reactants needed and the amounts of products formed. This information is vital in industrial processes, pharmaceutical manufacturing, and environmental science, among other areas. Furthermore, understanding how to balance equations helps in predicting the outcomes of reactions, identifying potential hazards, and optimizing reaction conditions for efficiency and safety.
What is the importance of balancing chemical equations?
+Balancing chemical equations is crucial because it ensures that the law of conservation of mass is upheld, allowing for accurate calculations of reactant and product quantities, which is essential in various chemical and industrial processes.
How do I choose the best method for balancing an equation?
+The choice of method depends on the complexity of the equation and personal preference. Simple equations can often be balanced by inspection, while more complex equations may require the algebraic method or the use of oxidation numbers.
What are some common challenges in balancing equations?
+Common challenges include dealing with complex reactions involving multiple steps or species, ensuring that all elements are properly accounted for, and avoiding common pitfalls such as altering the formulas of compounds rather than adding coefficients.
In conclusion, balancing equations is a foundational skill in chemistry that can be approached through various methods, each with its strengths and suitable applications. Whether through inspection, algebraic manipulation, or the use of oxidation numbers, the key to mastering the balance of equations lies in practice, patience, and a deep understanding of the underlying chemical principles. As one delves deeper into the world of chemistry, the ability to balance equations with ease and accuracy becomes an indispensable tool, facilitating the exploration of the intricate and fascinating world of chemical reactions.