The question of whether BF3 (boron trifluoride) is polar or nonpolar is a fundamental one in chemistry, particularly in the context of understanding molecular geometry and the distribution of electrons within a molecule. To address this, we must first consider the molecular structure of BF3 and the principles that determine polarity in molecules.
Molecular Structure of BF3
BF3 is composed of one boron atom bonded to three fluorine atoms. Boron, with an atomic number of 5, has three valence electrons, and each fluorine atom, with an atomic number of 9, has seven valence electrons. In the molecule, boron shares its three electrons with three fluorine atoms in covalent bonds, and each fluorine atom shares one of its seven valence electrons with boron, resulting in three single covalent bonds. This leaves each fluorine atom with six non-bonding electrons (three lone pairs), while boron has no lone pairs, having shared all its valence electrons.
Molecular Geometry
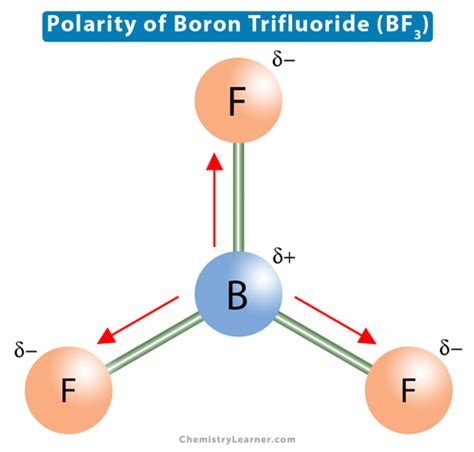
The molecular geometry of BF3 is trigonal planar, with the boron atom at the center and the three fluorine atoms at the vertices of an equilateral triangle. This geometry is a result of the sp2 hybridization of the boron atom, which allows the molecule to achieve the lowest possible energy state by maximizing the distance between the bonded pairs of electrons. In a trigonal planar arrangement, the bond angles are 120 degrees, and the molecule is symmetrical.
Determining Polarity
A molecule is considered polar if it has a net dipole moment, meaning there is a separation of positive and negative charges. This typically occurs in molecules with a asymmetrical distribution of electrons, often due to a difference in electronegativity between the atoms in a bond. Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond.
Electronegativity Considerations
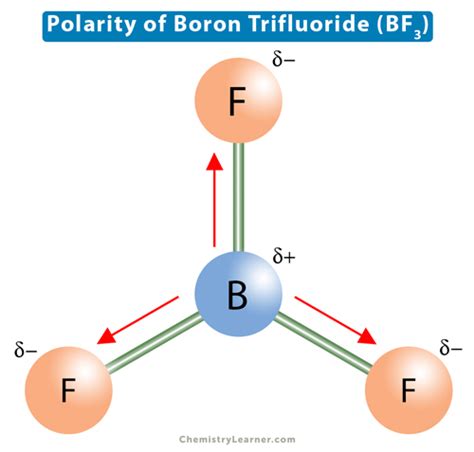
In BF3, boron has an electronegativity value of about 2.0, while fluorine has an electronegativity value of about 4.0. The significant difference in electronegativity between boron and fluorine suggests that each B-F bond is polar, with the fluorine atom pulling electrons closer to itself, creating a partial negative charge on fluorine and a partial positive charge on boron.
Conclusion on Polarity
However, despite the individual B-F bonds being polar, the overall molecule BF3 is nonpolar. This is because the molecule’s trigonal planar geometry is symmetrical, and the dipole moments of the individual B-F bonds cancel each other out. Since the three fluorine atoms are arranged symmetrically around the boron atom, the net dipole moment of the molecule is zero. Therefore, BF3 is considered a nonpolar molecule, even though it contains polar bonds.
Key Points
- BF3 has a trigonal planar molecular geometry due to sp2 hybridization of the boron atom.
- Each B-F bond is polar due to the difference in electronegativity between boron and fluorine.
- The symmetry of the trigonal planar geometry results in the cancellation of dipole moments of individual B-F bonds.
- BF3 is considered a nonpolar molecule despite containing polar bonds.
- The molecular polarity is determined by the overall distribution of electrons and the symmetry of the molecule.
| Property | Value |
|---|---|
| Molecular Geometry | Trigonal Planar |
| Boron Electronegativity | 2.0 |
| Fluorine Electronegativity | 4.0 |
| Polarity of B-F Bonds | Polar |
| Overall Molecule Polarity | Nonpolar |
What determines the polarity of a molecule?
+The polarity of a molecule is determined by the distribution of electrons within the molecule and the symmetry of its molecular geometry. A molecule is polar if it has a net dipole moment, which occurs when there is an asymmetrical distribution of electrons, often due to differences in electronegativity between atoms in a bond.
Why is BF3 considered nonpolar despite having polar bonds?
+BF3 is considered nonpolar because its trigonal planar geometry is symmetrical, and the dipole moments of the individual B-F bonds cancel each other out. Although each B-F bond is polar due to the difference in electronegativity between boron and fluorine, the symmetrical arrangement of these bonds results in a net dipole moment of zero for the molecule.