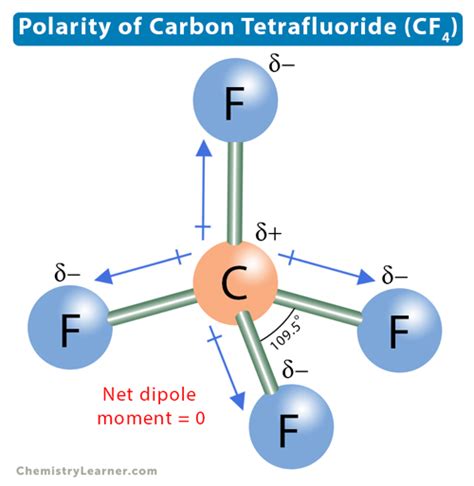
The question of whether CF4 is polar or nonpolar is a fundamental concept in chemistry, particularly in the realm of molecular geometry and polarity. To answer this question, we need to delve into the molecular structure of CF4, also known as carbon tetrafluoride, and understand the principles that determine the polarity of a molecule.
Molecular Structure of CF4
CF4 is composed of one carbon atom bonded to four fluorine atoms. The carbon atom is situated at the center, and the four fluorine atoms are arranged around it in a tetrahedral geometry. This arrangement is due to the sp3 hybridization of the carbon atom, which results in four equivalent orbitals that are directed towards the corners of a regular tetrahedron. The bond length between carbon and each fluorine atom is approximately 1.35 angstroms.
Determining Polarity
A molecule is considered polar if it has a net dipole moment, meaning there is a separation of positive and negative charges within the molecule. The polarity of a molecule is influenced by the difference in electronegativity between the atoms in a bond and the shape of the molecule. Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond.
In the case of CF4, the carbon atom is bonded to four fluorine atoms, which are highly electronegative. However, the symmetry of the tetrahedral shape is crucial in determining the polarity of CF4. Because the four fluorine atoms are arranged symmetrically around the central carbon atom, the individual bond dipoles (the vector sum of the electronegativity differences between carbon and each fluorine) cancel each other out.
| Atom | Electronegativity |
|---|---|
| Carbon (C) | 2.55 |
| Fluorine (F) | 3.98 |
This cancellation occurs because each bond dipole is of equal magnitude and is oriented in such a way that they point to the vertices of a regular tetrahedron, resulting in no net dipole moment for the molecule. Therefore, despite the significant difference in electronegativity between carbon and fluorine, the symmetrical arrangement of the fluorine atoms around the carbon atom leads to CF4 being classified as a nonpolar molecule.
Key Points

Key Points
- CF4 has a tetrahedral molecular geometry due to the sp3 hybridization of the carbon atom.
- The molecule is composed of one carbon atom bonded to four fluorine atoms.
- Despite the difference in electronegativity between carbon and fluorine, CF4 is nonpolar due to its symmetrical shape.
- The tetrahedral arrangement of the fluorine atoms around the carbon atom results in the cancellation of individual bond dipoles, leading to no net dipole moment.
- Understanding the molecular geometry and electronegativity differences is crucial in determining the polarity of a molecule.
In conclusion, CF4 is nonpolar due to its symmetrical tetrahedral shape, which causes the bond dipoles between the carbon and fluorine atoms to cancel each other out, resulting in no net dipole moment. This understanding is vital in chemistry, as it influences the physical and chemical properties of molecules, including their boiling points, solubility, and reactivity.
What is the primary factor that determines the polarity of a molecule like CF4?
+The primary factor is the molecular geometry and the difference in electronegativity between the atoms in the bonds. However, in the case of CF4, its tetrahedral symmetry is key to its nonpolarity.
Can a molecule with polar bonds be nonpolar?
+Yes, if the molecule has a symmetrical shape, like CF4, the polar bonds can cancel each other out, resulting in a nonpolar molecule.
How does the electronegativity of fluorine affect the polarity of CF4?
+Fluorine’s high electronegativity creates a significant difference in electronegativity between carbon and fluorine, which would normally result in polar bonds. However, due to the symmetrical arrangement of fluorine atoms in CF4, these polar bonds cancel each other out.