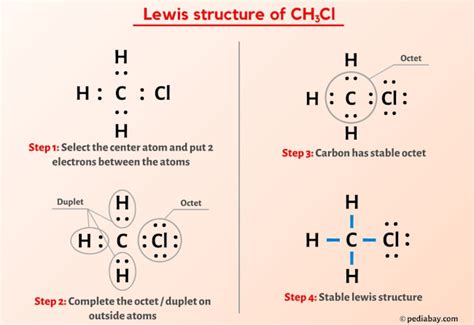
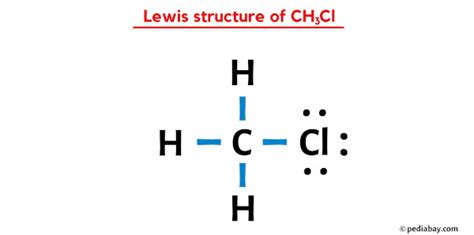
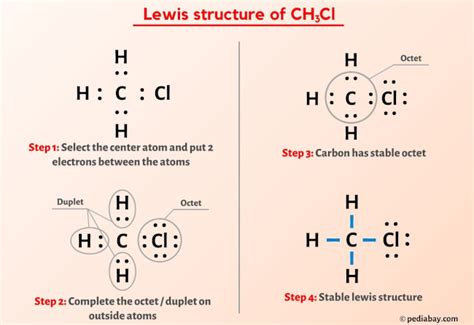
The CH3CL Lewis structure is a fundamental concept in organic chemistry, representing the molecular structure of chloromethane, also known as methyl chloride. To understand this structure, it's essential to have a basic knowledge of chemistry, including the concepts of atomic orbitals, electron configuration, and chemical bonding. In this article, we'll delve into the world of CH3CL, exploring its Lewis structure, properties, and significance in the realm of organic chemistry.
Key Points
- The CH3CL Lewis structure consists of a central carbon atom bonded to three hydrogen atoms and one chlorine atom.
- The carbon atom in CH3CL has a tetrahedral geometry, with the chlorine atom occupying one of the tetrahedral positions.
- The CH3CL molecule has a polar covalent bond between the carbon and chlorine atoms, resulting in a net dipole moment.
- CH3CL is a potent greenhouse gas and a toxic substance that requires handling with caution.
- The Lewis structure of CH3CL is essential in understanding its chemical properties, reactivity, and potential applications.
Understanding the CH3CL Lewis Structure
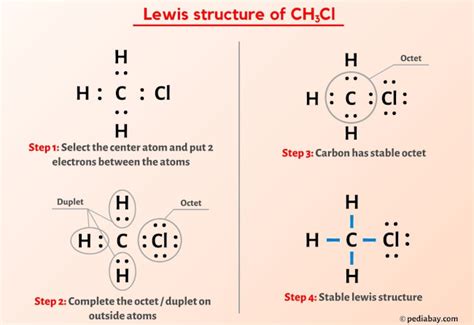
The Lewis structure of CH3CL can be drawn by following a series of steps. First, we need to determine the total number of valence electrons in the molecule. Carbon has four valence electrons, hydrogen has one, and chlorine has seven. The total number of valence electrons in CH3CL is 4 © + 3(1) (H) + 7 (Cl) = 14. Next, we draw the skeletal structure of the molecule, with the carbon atom at the center and the hydrogen and chlorine atoms surrounding it. We then distribute the valence electrons around the atoms, ensuring that each atom has a full outer shell of electrons, except for the carbon atom, which forms four bonds.
Drawing the CH3CL Lewis Structure
To draw the Lewis structure of CH3CL, we start by placing the carbon atom at the center. We then add the three hydrogen atoms and the chlorine atom around the carbon atom. The carbon atom forms four bonds: three with the hydrogen atoms and one with the chlorine atom. The chlorine atom has three lone pairs of electrons, which are not involved in bonding. The resulting structure shows a tetrahedral arrangement of atoms around the carbon atom, with the chlorine atom occupying one of the tetrahedral positions.
| Atom | Number of Valence Electrons | Number of Bonds |
|---|---|---|
| Carbon (C) | 4 | 4 |
| Hydrogen (H) | 1 | 1 |
| Chlorine (Cl) | 7 | 1 |
Properties of CH3CL
CH3CL is a colorless, toxic, and flammable gas with a characteristic sweet odor. It has a molecular weight of 50.49 g/mol and a boiling point of -24.2°C. The molecule has a polar covalent bond between the carbon and chlorine atoms, resulting in a net dipole moment of 1.86 D. This polarity makes CH3CL a useful solvent in various chemical reactions.
Chemical Reactions of CH3CL
CH3CL is a versatile molecule that participates in various chemical reactions. It can undergo substitution reactions, where the chlorine atom is replaced by another atom or group. It can also undergo elimination reactions, where the chlorine atom is removed, resulting in the formation of a new bond. Additionally, CH3CL can react with bases, such as sodium hydroxide, to form methanol and sodium chloride.
What is the significance of the CH3CL Lewis structure in understanding its chemical properties?
+The CH3CL Lewis structure is essential in understanding the chemical properties of the molecule, including its polarity, boiling point, and reactivity. By analyzing the structure, we can predict the molecule's behavior in various chemical reactions and its potential applications.
What are the potential hazards associated with handling CH3CL?
+CH3CL is a toxic and flammable gas that requires handling with caution. It can cause respiratory problems, skin irritation, and eye damage. Prolonged exposure to CH3CL can also lead to more severe health problems, including cancer and neurological damage.
What are the potential applications of CH3CL in various industries?
+CH3CL has various applications in different industries, including the production of pharmaceuticals, agrochemicals, and refrigerants. It is also used as a solvent in chemical reactions and as a fuel in some industrial processes.
In conclusion, the CH3CL Lewis structure is a fundamental concept in organic chemistry that helps us understand the molecular structure and properties of chloromethane. By analyzing the structure, we can predict the molecule’s behavior in various chemical reactions and its potential applications. However, it’s essential to handle CH3CL with caution due to its toxic and flammable nature. As we continue to explore the properties and applications of CH3CL, we must prioritize safety and responsible handling to minimize its potential hazards.