The proton charge is a fundamental concept in physics, particularly in the realm of particle physics and chemistry. Understanding the proton charge is crucial for grasping the structure of atoms, the properties of molecules, and the behavior of matter at its most basic level. Here are five key facts about the proton charge, delving into its nature, magnitude, and implications.
Introduction to Proton Charge
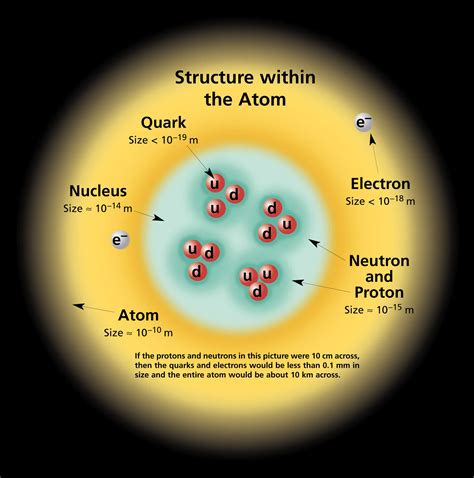
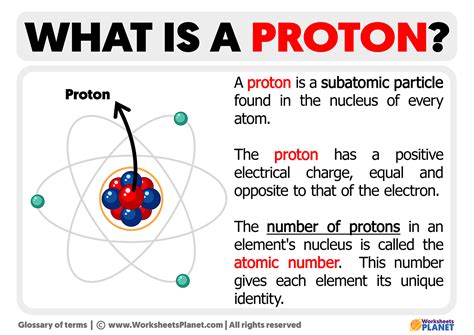
The proton is one of the three main subatomic particles that make up atoms, alongside electrons and neutrons. Protons reside in the nucleus of an atom, which is the central part of the atom, while electrons orbit around the nucleus. Each proton carries a positive charge, and the number of protons in an atom’s nucleus determines the element of the atom. For instance, hydrogen has one proton, helium has two, and so on.
Fact 1: Magnitude of Proton Charge
The charge of a proton is a well-defined physical constant. It is approximately equal to 1.60217662 × 10^-19 coulombs. This value is a fundamental constant in physics and is denoted by the symbol e. The proton’s positive charge is exactly balanced by the negative charge of an electron, which also carries a charge of -e. This balance is crucial for the formation of neutral atoms and molecules.
Fact 2: Stability of Proton Charge
One of the most intriguing aspects of the proton charge is its stability. Experiments and observations have shown that the proton is a remarkably stable particle. Its half-life, which is the time it takes for half of a sample of protons to decay, is estimated to be at least 10^31 years, possibly even infinite. This stability is crucial for the existence of matter as we know it, as protons form the backbone of atomic nuclei.
Fact 3: Role in Atomic Structure
The proton charge plays a pivotal role in determining the structure of atoms. The positive charge of protons attracts negatively charged electrons, leading to the formation of electron orbits or shells around the nucleus. The number of protons (atomic number) defines the chemical element and determines how many electrons an atom can have. This, in turn, influences the chemical properties of an element, such as its reactivity and the types of compounds it can form.
Fact 4: Implications for Chemistry
The proton charge has profound implications for chemistry. The interaction between protons and electrons is the basis of chemical bonding. Ionic bonds form when electrons are transferred between atoms, leading to the formation of ions with opposite charges that attract each other. Covalent bonds, on the other hand, involve the sharing of electron pairs between atoms, with the proton charges influencing the distribution of electrons and thus the strength and nature of these bonds.
Fact 5: Experimental Verification
The value and implications of the proton charge have been extensively verified through various experiments. Millikan’s oil drop experiment, conducted in the early 20th century, was one of the first to directly measure the charge of an electron (and by inference, a proton). Since then, numerous experiments in particle physics, including those using particle accelerators, have further refined our understanding of proton properties, including its charge.
Key Points
- The proton charge is approximately 1.60217662 × 10^-19 coulombs, a fundamental constant in physics.
- Protons are stable particles with a half-life of at least 10^31 years, ensuring the stability of matter.
- The proton charge determines the chemical properties of an element by influencing electron distribution and chemical bonding.
- The interaction between protons and electrons is the basis for all chemical reactions and the formation of compounds.
- Experimental verification, including Millikan's oil drop experiment, has confirmed the proton charge and its implications for physics and chemistry.
In conclusion, the proton charge is a cornerstone of physics and chemistry, underpinning our understanding of atomic structure, chemical bonding, and the behavior of matter. Its precise value and stability are fundamental to the existence of atoms and molecules as we understand them, and ongoing research continues to refine our knowledge of this and other physical constants.
What is the significance of the proton charge in chemistry?
+The proton charge is crucial in chemistry as it influences the formation of chemical bonds. The positive charge of protons attracts electrons, leading to the sharing or transfer of electrons between atoms and the formation of ionic and covalent bonds.
How is the proton charge measured?
+The proton charge has been measured through various experiments, most notably Millikan’s oil drop experiment. This experiment involved measuring the charge on tiny oil droplets suspended between two metal plates, allowing for the calculation of the elementary charge, which is the charge of a proton.
What would happen if the proton charge were different?
+If the proton charge were different, it would significantly alter the structure of atoms and molecules. Changes in the proton charge would affect the stability of nuclei, the formation of chemical bonds, and thus the properties of elements and compounds. This, in turn, would have profound implications for the existence and diversity of matter in the universe.