The charge of chlorine is a fundamental concept in chemistry, particularly in the realm of inorganic chemistry and electrochemistry. Chlorine, with the atomic number 17, is a member of the halogen family in the periodic table. Understanding the charge of chlorine is crucial for grasping its reactivity, the formation of compounds, and its participation in chemical reactions. In its elemental form, chlorine is a diatomic molecule (Cl2), where each chlorine atom shares a covalent bond, resulting in a zero overall charge for the molecule.
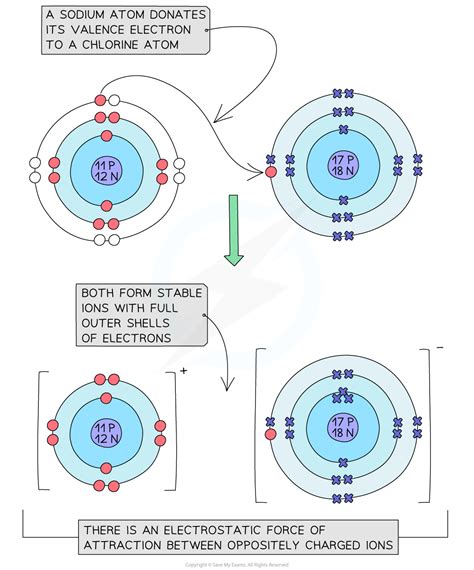
However, when chlorine reacts with other elements to form compounds, the charge it carries can vary significantly. Chlorine can exhibit several oxidation states, ranging from -1 to +7, depending on the compound it forms. The most common oxidation state of chlorine is -1, as seen in chloride ions (Cl-), which are formed when chlorine gains an electron. This -1 charge is characteristic of chlorides, such as sodium chloride (NaCl) or calcium chloride (CaCl2), where chlorine is in its most reduced state.
Key Points
- The charge of chlorine in its elemental form is zero.
- Chlorine can exhibit several oxidation states, including -1, +1, +3, +5, and +7.
- The most common oxidation state of chlorine is -1, as seen in chloride ions (Cl-).
- Chlorine's charge is crucial for understanding its reactivity and the formation of compounds.
- Understanding the charge of chlorine is essential in various chemical and industrial processes.
Oxidation States of Chlorine
Chlorine’s ability to exhibit multiple oxidation states makes it a versatile element in chemical reactions. Besides the -1 state in chlorides, chlorine can also be found in compounds with higher oxidation states, such as in chlorates (ClO3-), where chlorine has an oxidation state of +5, or in perchlorates (ClO4-), where the oxidation state is +7. These higher oxidation states are indicative of chlorine’s ability to act as an oxidizing agent, accepting electrons to form compounds with lower oxidation states.
Chemical Reactivity of Chlorine
The chemical reactivity of chlorine is significantly influenced by its charge. In its elemental form, chlorine is highly reactive due to its strong tendency to gain an electron and form a chloride ion. This reactivity is the basis for chlorine’s use as a disinfectant and its role in various industrial processes, including the production of plastics, dyes, and pharmaceuticals. The charge of chlorine also plays a critical role in its participation in redox reactions, where the transfer of electrons results in a change in the oxidation state of chlorine.
| Oxidation State | Compound Example | Description |
|---|---|---|
| -1 | Sodium Chloride (NaCl) | Common table salt, where chlorine is in its most reduced state. |
| +5 | Sodium Chlorate (NaClO3) | Used in the production of matches and as a herbicide, illustrating chlorine's higher oxidation state. |
| +7 | Sodium Perchlorate (NaClO4) | Used in rocket propulsion, demonstrating chlorine's highest oxidation state. |
Industrial Applications and Environmental Considerations
The charge of chlorine has significant implications for its industrial applications and environmental impact. Chlorine’s role as a disinfectant in water treatment, for example, relies on its ability to accept electrons and form hypochlorous acid, which is effective against a wide range of pathogens. However, the use of chlorine in industrial processes also raises environmental concerns, such as the potential for the formation of harmful by-products, like dioxins, when chlorine reacts with organic materials.
In conclusion, the charge of chlorine is a critical aspect of its chemical behavior, influencing its reactivity, the formation of compounds, and its participation in chemical reactions. Understanding the various oxidation states of chlorine and their implications is essential for harnessing its utility in industrial processes while minimizing its environmental impact.
What is the most common oxidation state of chlorine?
+The most common oxidation state of chlorine is -1, as seen in chloride ions (Cl-), which are formed when chlorine gains an electron.
Why is the charge of chlorine important in chemical reactions?
+The charge of chlorine is crucial for understanding its reactivity and the formation of compounds. It influences how chlorine participates in redox reactions and its ability to form bonds with other elements.
What are some common compounds where chlorine exhibits higher oxidation states?
+Chlorine exhibits higher oxidation states in compounds such as chlorates (ClO3-), where its oxidation state is +5, and perchlorates (ClO4-), where its oxidation state is +7. These compounds are used in various applications, including as oxidizing agents and in rocket propulsion.