Chemical weathering is a fundamental process that shapes our planet's surface, breaking down rocks and minerals into smaller components through chemical reactions. This process is driven by the interaction of rocks with water, air, and living organisms, leading to the formation of new minerals and the release of nutrients essential for life. Understanding chemical weathering is crucial for grasping the Earth's geological cycles, including the carbon cycle, and for managing natural resources sustainably. In this article, we will delve into the world of chemical weathering, exploring its mechanisms, examples, and significance in shaping our environment.
Key Points
- Chemical weathering involves the breakdown of rocks through chemical reactions with water, air, and biological agents.
- There are several types of chemical weathering, including hydrolysis, oxidation, carbonation, and dissolution.
- Examples of chemical weathering include the formation of karst landscapes, the weathering of granite into sand, and the decomposition of limestone.
- Chemical weathering plays a critical role in the Earth's geological and biological cycles, influencing soil formation, water quality, and ecosystem health.
- Human activities, such as pollution and climate change, can accelerate chemical weathering, with significant implications for environmental sustainability.
Types of Chemical Weathering

Chemical weathering can be categorized into several types, each involving distinct chemical reactions that alter the composition of rocks. Hydrolysis is one of the most common forms, where water reacts with minerals to form new minerals and release ions. Oxidation occurs when minerals react with oxygen, leading to the formation of oxides and a change in the mineral’s color and composition. Carbonation is another important process, where carbon dioxide in rainwater reacts with minerals like calcium carbonate to form soluble bicarbonates, contributing to the dissolution of rocks like limestone. Finally, dissolution involves the direct dissolution of rocks in water, which is particularly significant for soluble minerals like halite (rock salt) and gypsum.
Examples of Chemical Weathering
One of the most striking examples of chemical weathering is the formation of karst landscapes. These unique landscapes are characterized by caves, sinkholes, and underground rivers, all formed through the dissolution of soluble rocks like limestone and dolomite by acidic rainwater. The process begins with the reaction of carbon dioxide from the air with water to form carbonic acid, a weak acid that can dissolve limestone. Over time, this dissolution creates passages and caverns, eventually leading to the collapse of the rock surface and the formation of characteristic karst features.
| Type of Chemical Weathering | Chemical Reaction | Example |
|---|---|---|
| Hydrolysis | Mineral + Water → New Mineral + Ions | Weathering of feldspar to form clay minerals |
| Oxidation | Mineral + Oxygen → Oxide Mineral | Rusting of iron-rich rocks |
| Carbonation | Calcium Carbonate + Carbon Dioxide + Water → Calcium Bicarbonate | Dissolution of limestone |
| Dissolution | Mineral + Water → Dissolved Ions | Dissolution of rock salt (halite) in water |

Significance of Chemical Weathering
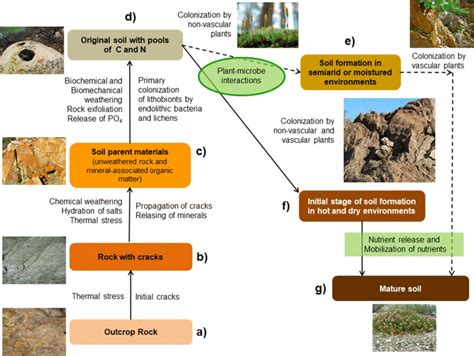
Chemical weathering is not just a geological process; it has profound implications for the Earth’s ecosystems and human societies. It plays a critical role in soil formation, as the breakdown of rocks releases nutrients essential for plant growth. Chemical weathering also influences water quality, as the dissolution of rocks can lead to the release of ions and minerals into water bodies, affecting their chemistry and the organisms that depend on them. Furthermore, chemical weathering is closely linked to the carbon cycle, as it helps to regulate the concentration of carbon dioxide in the atmosphere by removing it through the formation of carbonate minerals.
Human Impact on Chemical Weathering
Human activities can significantly accelerate chemical weathering, with potentially detrimental environmental consequences. Pollution, for example, can introduce acidic compounds into the environment, enhancing the weathering rate of rocks. Climate change also plays a role, as increased temperatures and altered precipitation patterns can accelerate chemical reactions and the breakdown of rocks. Understanding these impacts is essential for developing strategies to mitigate environmental degradation and ensure the long-term sustainability of our planet’s resources.
What is the primary driver of chemical weathering?
+The primary driver of chemical weathering is the interaction of rocks with water, air, and living organisms, which leads to chemical reactions that break down the rocks.
How does human activity influence chemical weathering?
+Human activities such as pollution and climate change can accelerate chemical weathering by introducing acidic compounds into the environment and altering temperature and precipitation patterns.
What is the significance of chemical weathering in ecosystems?
+Chemical weathering plays a critical role in soil formation, influences water quality, and is linked to the carbon cycle, making it essential for ecosystem health and sustainability.
In conclusion, chemical weathering is a complex and multifaceted process that underpins many of the Earth’s geological and biological systems. Through its various types and examples, chemical weathering shapes our landscapes, influences our ecosystems, and impacts our daily lives. As we move forward in an era of environmental change, understanding and managing chemical weathering will be crucial for conserving natural resources, mitigating the effects of pollution and climate change, and ensuring the long-term health of our planet.