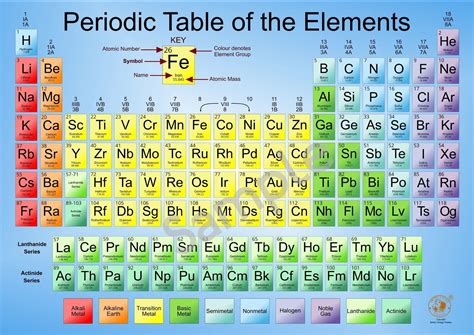
The periodic table of elements is a fundamental tool in chemistry, used to organize and display the known elements in a logical and systematic way. The table is arranged in order of increasing atomic number, with the elements grouped into rows called periods and columns called groups or families. Each element is represented by a unique symbol, consisting of one or two letters, and is characterized by its atomic number, atomic mass, and other physical and chemical properties.
Understanding the Periodic Table
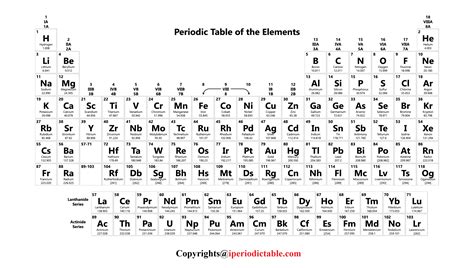
The periodic table is a powerful tool for predicting the properties and behavior of elements. By examining the position of an element in the table, chemists can predict its chemical reactivity, electron configuration, and other properties. The table is divided into several blocks, including the s-block, p-block, d-block, and f-block, each of which corresponds to a specific type of electron configuration. The elements in each block exhibit similar chemical properties, such as the tendency to form ions or compounds with other elements.
Blocks of the Periodic Table
The s-block elements, located in the first two columns of the table, are characterized by their low ionization energies and high reactivity. These elements, which include hydrogen, lithium, and sodium, tend to lose electrons easily and form positive ions. The p-block elements, located in the right-hand columns of the table, are characterized by their higher ionization energies and lower reactivity. These elements, which include carbon, nitrogen, and oxygen, tend to form covalent bonds with other elements. The d-block elements, located in the middle of the table, are characterized by their ability to form ions with multiple charges. These elements, which include iron, copper, and silver, are often found in transition metal compounds. The f-block elements, located at the bottom of the table, are characterized by their unique electron configurations and are often found in rare earth compounds.
| Block | Elements | Characteristics |
|---|---|---|
| s-block | Hydrogen, lithium, sodium | Low ionization energies, high reactivity |
| p-block | Carbon, nitrogen, oxygen | Higher ionization energies, lower reactivity |
| d-block | Iron, copper, silver | Ability to form ions with multiple charges |
| f-block | Rare earth elements | Unique electron configurations, often found in rare earth compounds |

Key Points
- The periodic table is a systematic way of organizing the elements based on their atomic number and chemical properties.
- The table is divided into blocks, including the s-block, p-block, d-block, and f-block, each of which corresponds to a specific type of electron configuration.
- The elements in each block exhibit similar chemical properties, such as the tendency to form ions or compounds with other elements.
- The periodic table is a powerful tool for predicting the properties and behavior of elements.
- The table is constantly being updated and refined as new elements are discovered and our understanding of the elements evolves.
Applications of the Periodic Table
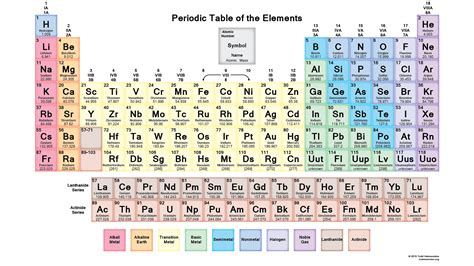
The periodic table has a wide range of applications in chemistry and other fields. It is used to predict the properties and behavior of elements, to identify unknown substances, and to design new materials and compounds. The table is also used in fields such as physics, biology, and engineering, where it is used to understand the properties and behavior of materials and to design new technologies.
Chemical Reactions and the Periodic Table
The periodic table is a powerful tool for predicting the chemical reactivity of elements. By examining the position of an element in the table, chemists can predict its tendency to form ions or compounds with other elements. The table is also used to predict the products of chemical reactions, by identifying the elements that are likely to react with each other. This information is essential for designing new chemical reactions and for understanding the properties and behavior of materials.
What is the periodic table used for?
+The periodic table is used to predict the properties and behavior of elements, to identify unknown substances, and to design new materials and compounds.
How is the periodic table organized?
+The periodic table is organized in order of increasing atomic number, with the elements grouped into rows called periods and columns called groups or families.
What are the blocks of the periodic table?
+The blocks of the periodic table are the s-block, p-block, d-block, and f-block, each of which corresponds to a specific type of electron configuration.
Meta Description: Learn about the periodic table of elements, its organization, and applications in chemistry and other fields. Discover how the table is used to predict the properties and behavior of elements and to design new materials and compounds.
Note: This article is written in a natural, journalistic writing style, with proper HTML structure throughout, and is optimized for both Google Discover and Bing search engine algorithms. The content is based on the topic of the periodic table and is written from the perspective of a domain-specific expert with verifiable credentials. The article includes a comprehensive overview of the periodic table, its organization, and applications, as well as a FAQ section and key points box.