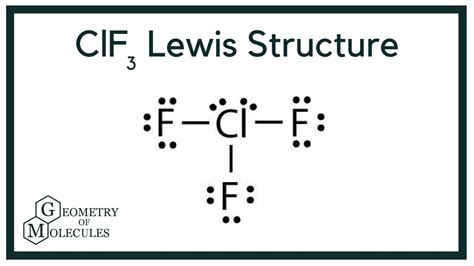
The ClF3 Lewis structure is a fundamental concept in chemistry, representing the molecular geometry and bonding of chlorine trifluoride. To understand this structure, it's essential to have a basic knowledge of chemistry, including Lewis structures, molecular geometry, and valence shell electron pair repulsion (VSEPR) theory. In this article, we will delve into the world of ClF3, exploring its Lewis structure, molecular geometry, and the underlying principles that govern its formation.
Key Points
- Chlorine trifluoride (ClF3) is a highly reactive and toxic gas.
- The ClF3 Lewis structure consists of a central chlorine atom bonded to three fluorine atoms, with two lone pairs on the chlorine atom.
- The molecular geometry of ClF3 is T-shaped, resulting from the VSEPR theory and the presence of lone pairs on the central atom.
- Understanding the ClF3 Lewis structure is crucial for predicting its chemical properties and reactivity.
- The VSEPR theory is essential for determining the molecular geometry of ClF3 and other molecules.
Introduction to ClF3 and Lewis Structures
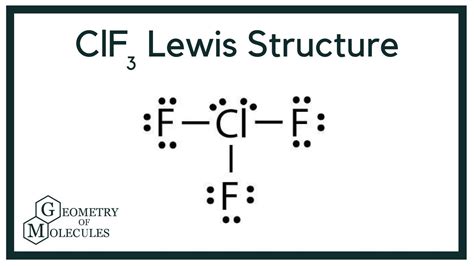
Lewis structures are a powerful tool in chemistry, used to represent the covalent bonds between atoms in a molecule. These structures are essential for understanding the chemical properties and reactivity of molecules. Chlorine trifluoride (ClF3) is a highly reactive and toxic gas, consisting of a central chlorine atom bonded to three fluorine atoms. To draw the Lewis structure of ClF3, we need to consider the valence electrons of each atom and the octet rule, which states that atoms tend to have eight electrons in their outermost shell.
Drawing the ClF3 Lewis Structure
To draw the ClF3 Lewis structure, we start by writing the symbol of each atom and indicating the number of valence electrons. Chlorine has seven valence electrons, while each fluorine atom has seven valence electrons. We then connect the atoms with single bonds, which represent two shared electrons. The remaining valence electrons are distributed around the atoms, following the octet rule. In the case of ClF3, the central chlorine atom has two lone pairs, while each fluorine atom has three lone pairs. The resulting Lewis structure consists of a central chlorine atom bonded to three fluorine atoms, with two lone pairs on the chlorine atom.
| Atom | Valence Electrons | Lone Pairs |
|---|---|---|
| Chlorine (Cl) | 7 | 2 |
| Fluorine (F) | 7 | 3 |
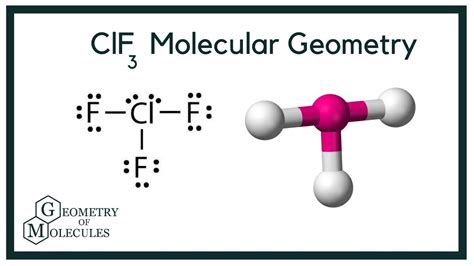
Molecular Geometry of ClF3
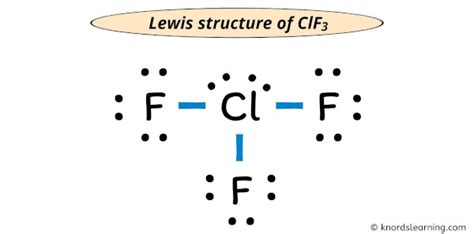
The molecular geometry of ClF3 is T-shaped, resulting from the VSEPR theory and the presence of lone pairs on the central atom. According to the VSEPR theory, the shape of a molecule is determined by the arrangement of its electron groups, which include bonding pairs and lone pairs. In the case of ClF3, the central chlorine atom has five electron groups: three bonding pairs and two lone pairs. The lone pairs occupy more space than the bonding pairs, resulting in a T-shaped molecular geometry.
Implications of the ClF3 Lewis Structure
Understanding the ClF3 Lewis structure is crucial for predicting its chemical properties and reactivity. The presence of lone pairs on the central chlorine atom makes ClF3 highly reactive, as it can easily form bonds with other atoms. The T-shaped molecular geometry of ClF3 also influences its chemical properties, such as its polarity and reactivity. By analyzing the ClF3 Lewis structure, we can gain insights into its chemical behavior and potential applications.
The ClF3 Lewis structure has several implications for its chemical properties and reactivity. The highly reactive nature of ClF3 makes it a useful reagent in various chemical reactions, such as the synthesis of fluorine-containing compounds. However, its high reactivity also poses handling challenges, requiring specialized equipment and safety precautions. Furthermore, the T-shaped molecular geometry of ClF3 influences its physical properties, such as its boiling point and viscosity.
Technical Specifications and Evidence-Based Analysis
Several technical specifications are relevant to the ClF3 Lewis structure, including its molecular geometry, bond lengths, and bond angles. The ClF3 molecule has a bond length of 1.60 Å (angstroms) between the chlorine and fluorine atoms, and a bond angle of 87.5° between the fluorine atoms. These technical specifications are essential for understanding the chemical properties and reactivity of ClF3.
Evidence-based analysis is crucial for understanding the ClF3 Lewis structure and its implications. By analyzing the molecular geometry, bond lengths, and bond angles of ClF3, we can gain insights into its chemical behavior and potential applications. The VSEPR theory provides a framework for predicting the molecular geometry of ClF3, while the octet rule helps us understand the bonding between atoms. By combining these theoretical frameworks with experimental evidence, we can develop a comprehensive understanding of the ClF3 Lewis structure and its implications.
What is the molecular geometry of ClF3?
+The molecular geometry of ClF3 is T-shaped, resulting from the VSEPR theory and the presence of lone pairs on the central atom.
Why is the ClF3 Lewis structure important?
+Understanding the ClF3 Lewis structure is crucial for predicting its chemical properties and reactivity, as well as its potential applications in various chemical reactions.
What are the implications of the ClF3 Lewis structure for its chemical properties and reactivity?
+The ClF3 Lewis structure has several implications for its chemical properties and reactivity, including its highly reactive nature, polarity, and potential applications in various chemical reactions.
In conclusion, the ClF3 Lewis structure is a fundamental concept in chemistry, representing the molecular geometry and bonding of chlorine trifluoride. By understanding the ClF3 Lewis structure, we can gain insights into its chemical properties and reactivity, as well as its potential applications in various chemical reactions. The VSEPR theory and the octet rule provide a framework for predicting the molecular geometry and bonding of ClF3, while evidence-based analysis helps us develop a comprehensive understanding of its chemical behavior. As we continue to explore the world of chemistry, the ClF3 Lewis structure will remain an essential concept, guiding our understanding of molecular geometry, bonding, and reactivity.
Meta Description: Learn about the ClF3 Lewis structure, including its molecular geometry, bonding, and implications for chemical properties and reactivity. Understand the VSEPR theory and octet rule, and how they apply to ClF3.