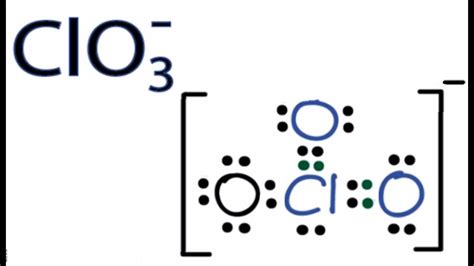The Chlorine Trioxide (ClO3) Lewis structure is a fundamental concept in chemistry, representing the molecular geometry and bonding pattern of the Chlorine Trioxide molecule. To draw the Lewis structure, we need to consider the valence electrons of each atom and the rules of Lewis structure drawing.
Understanding the Basics of Lewis Structures
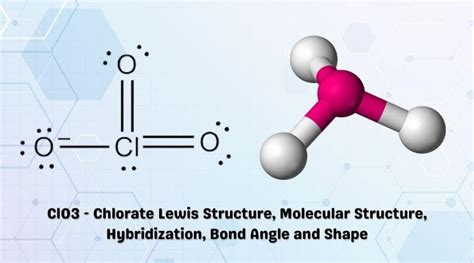
Lewis structures are two-dimensional representations of molecular structures, using dots to represent valence electrons and lines to represent covalent bonds. The Chlorine Trioxide molecule consists of one Chlorine atom and three Oxygen atoms. The Chlorine atom has 7 valence electrons, while each Oxygen atom has 6 valence electrons.
Calculating the Total Valence Electrons
To draw the Lewis structure, we need to calculate the total number of valence electrons in the molecule. The Chlorine atom contributes 7 valence electrons, and each Oxygen atom contributes 6 valence electrons, resulting in a total of 7 + (3 x 6) = 25 valence electrons.
| Atom | Valence Electrons |
|---|---|
| Chlorine (Cl) | 7 |
| Oxygen (O) | 6 |
| Total | 25 |
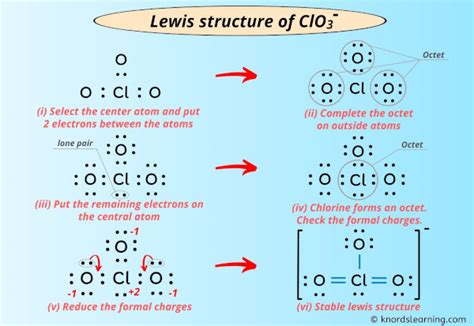
5 Ways to Draw the ClO3 Lewis Structure
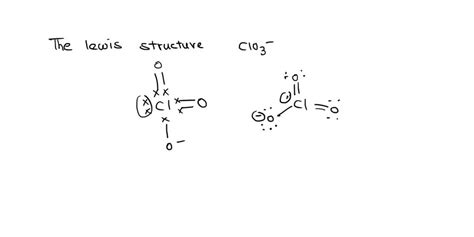
There are multiple ways to draw the Lewis structure of ClO3, each representing a possible resonance structure. Here are five common ways to draw the ClO3 Lewis structure:
Method 1: Single Bond Representation
In this method, we draw a single bond between the Chlorine atom and each Oxygen atom, resulting in a trigonal planar molecular geometry.
Step 1: Draw the Chlorine atom and three Oxygen atoms, with the Chlorine atom at the center.
Step 2: Draw a single bond between the Chlorine atom and each Oxygen atom.
Step 3: Distribute the remaining valence electrons to satisfy the octet rule for each atom.
Method 2: Double Bond Representation
In this method, we draw a double bond between the Chlorine atom and one Oxygen atom, and single bonds between the Chlorine atom and the remaining two Oxygen atoms.
Technical note: The double bond representation is more stable than the single bond representation, as it satisfies the octet rule for the Chlorine atom.
Method 3: Triple Bond Representation
In this method, we draw a triple bond between the Chlorine atom and one Oxygen atom, and single bonds between the Chlorine atom and the remaining two Oxygen atoms.
Step 1: Draw the Chlorine atom and three Oxygen atoms, with the Chlorine atom at the center.
Step 2: Draw a triple bond between the Chlorine atom and one Oxygen atom.
Step 3: Draw single bonds between the Chlorine atom and the remaining two Oxygen atoms.
Method 4: Resonance Hybrid Representation
In this method, we draw a resonance hybrid structure, which represents the average of multiple resonance structures.
Conceptual note: The resonance hybrid representation is a more accurate representation of the molecular structure, as it takes into account the delocalization of electrons.
Method 5: Expanded Octet Representation
In this method, we draw an expanded octet structure, which allows the Chlorine atom to have more than 8 valence electrons.
Step 1: Draw the Chlorine atom and three Oxygen atoms, with the Chlorine atom at the center.
Step 2: Draw a single bond between the Chlorine atom and each Oxygen atom.
Step 3: Distribute the remaining valence electrons to satisfy the expanded octet rule for the Chlorine atom.
Key Points
- The Chlorine Trioxide molecule has a trigonal planar molecular geometry.
- There are multiple ways to draw the ClO3 Lewis structure, each representing a possible resonance structure.
- The double bond representation is more stable than the single bond representation.
- The resonance hybrid representation is a more accurate representation of the molecular structure.
- The expanded octet representation allows the Chlorine atom to have more than 8 valence electrons.
What is the total number of valence electrons in the ClO3 molecule?
+The total number of valence electrons in the ClO3 molecule is 25.
What is the molecular geometry of the ClO3 molecule?
+The molecular geometry of the ClO3 molecule is trigonal planar.
Why is the double bond representation more stable than the single bond representation?
+The double bond representation is more stable than the single bond representation because it satisfies the octet rule for the Chlorine atom.