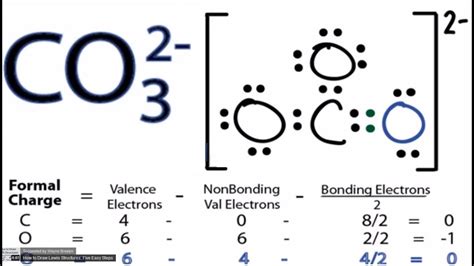
The CO32- ion, also known as the carbonate ion, is a polyatomic anion consisting of one carbon atom and three oxygen atoms. Understanding the Lewis structure of CO32- is essential for grasping its chemical properties and behavior. The Lewis structure is a two-dimensional representation of the molecule's electron configuration, showcasing how electrons are distributed among the atoms. Here, we will explore the CO32- Lewis structure in depth, considering the steps to draw it, its significance, and the implications of its structure on the ion's properties.
Understanding the Basics of Lewis Structures
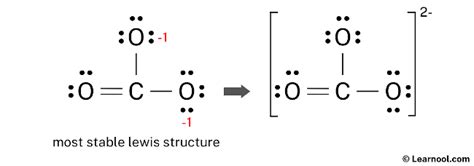
Lewis structures are drawn using a set of rules established by Gilbert N. Lewis. These rules help in predicting the structure of a molecule based on the number of valence electrons it has. For CO32-, we start by calculating the total number of valence electrons. Carbon has 4 valence electrons, each oxygen has 6, and since CO32- has a -2 charge, we add 2 more electrons to the total count, resulting in 4 (from C) + 3*6 (from 3 O) + 2 (from the charge) = 24 valence electrons.
Drawing the CO32- Lewis Structure
To draw the CO32- Lewis structure, we follow these steps:
- Step 1: Determine the Central Atom - Carbon is the least electronegative atom and thus becomes the central atom.
- Step 2: Calculate the Total Valence Electrons - As calculated, CO32- has 24 valence electrons.
- Step 3: Draw Single Bonds - We draw single bonds between the carbon and each of the three oxygens, which accounts for 6 electrons (2 electrons per bond).
- Step 4: Distribute Remaining Electrons - After forming the single bonds, we have 24 - 6 = 18 electrons left. These are distributed around the oxygens to satisfy the octet rule for each oxygen, resulting in 6 electrons (3 pairs) on each oxygen.
- Step 5: Achieve a Stable Structure - To achieve a stable structure where each atom has a full outer shell (octet), and considering the formal charges, we convert one of the single bonds to a double bond, giving one oxygen a single bond and a -1 formal charge, and the other two oxygens each having a single bond and sharing the remaining -1 charge evenly, leading to two oxygens with a single bond and a partial negative charge, and one oxygen with a double bond and no formal charge.
Resonance Structures
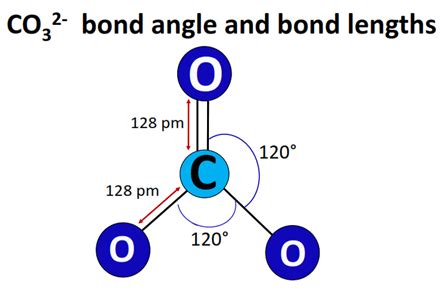
Since the double bond in the CO32- ion can be between the carbon and any of the three oxygens, we draw resonance structures to represent this delocalization of electrons. There are three main resonance structures for CO32-, each with a double bond between the carbon and a different oxygen. These structures are equivalent and contribute equally to the overall structure of the ion, indicating that the actual structure of CO32- is a hybrid of these resonance forms.
Implications of the CO32- Lewis Structure
The Lewis structure of CO32- has significant implications for its chemical properties. The delocalization of electrons due to resonance leads to increased stability of the ion, making it less reactive than it would be if the double bond were localized between the carbon and a single oxygen. This stability is crucial for the carbonate ion’s role in various chemical and biological processes, including its presence in minerals, its role in the carbon cycle, and its use in industrial applications.
| Property | Description |
|---|---|
| Electron Distribution | Delocalized electrons due to resonance |
| Chemical Reactivity | Stability due to delocalization reduces reactivity |
| Biological Role | Crucial in the carbon cycle and in various biological processes |

Key Points
- The CO32- ion has a total of 24 valence electrons, which are distributed to form single and double bonds between the carbon and oxygen atoms.
- The Lewis structure involves drawing single bonds between carbon and each oxygen and then distributing the remaining electrons to achieve a stable octet configuration for each atom.
- Resonance structures are used to represent the delocalization of the double bond among the three oxygen atoms, contributing to the ion's stability.
- The delocalization of electrons in CO32- leads to increased stability, affecting its chemical reactivity and biological roles.
- Understanding the CO32- Lewis structure is crucial for predicting its behavior in various chemical and biological contexts.
What is the significance of the CO32- Lewis structure in understanding its chemical properties?
+The CO32- Lewis structure is significant because it shows the distribution of electrons, which influences the ion’s reactivity, stability, and interactions with other molecules. The delocalization of electrons, as depicted by the resonance structures, contributes to the ion’s stability and reduces its reactivity.
How does the resonance in CO32- affect its biological role?
+The resonance in CO32- enhances its stability, which is crucial for its biological roles. The carbonate ion plays a vital role in the carbon cycle, and its stability allows it to participate effectively in these processes without readily decomposing or reacting inappropriately.
What are the steps to draw the CO32- Lewis structure?
+To draw the CO32- Lewis structure, start by determining the central atom (carbon), calculate the total valence electrons (24 for CO32-), draw single bonds between the carbon and each oxygen, distribute the remaining electrons to satisfy the octet rule for each atom, and finally, achieve a stable structure by considering formal charges and resonance.