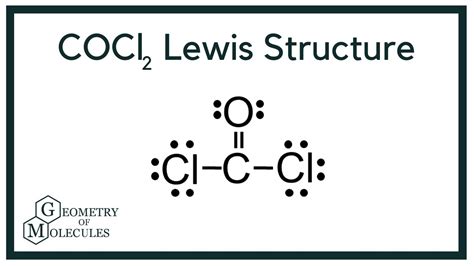
The COCl2 Lewis structure, also known as phosgene, is a crucial concept in chemistry, particularly in the realm of organic and inorganic compounds. Phosgene is a toxic gas that was once used as a chemical warfare agent, and its Lewis structure is essential for understanding its properties and reactivity. In this article, we will delve into the world of COCl2, exploring its Lewis structure, properties, and significance in the chemical industry.
Key Points
- The COCl2 Lewis structure consists of a central carbon atom bonded to one oxygen atom and two chlorine atoms.
- The carbon atom in COCl2 has a trigonal planar geometry, with bond angles of approximately 120 degrees.
- Phosgene is a highly toxic gas, with a threshold limit value (TLV) of 0.1 ppm in the workplace.
- COCl2 is synthesized through the reaction of carbon monoxide and chlorine gas.
- The compound has various applications in the chemical industry, including the production of polyurethane foams and pesticides.
COC12 Lewis Structure
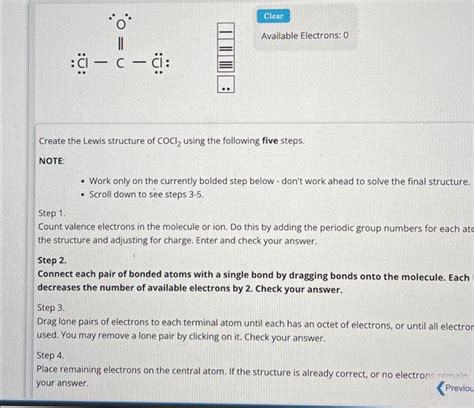
To draw the Lewis structure of COCl2, we need to follow a series of steps. First, we determine the total number of valence electrons in the molecule, which is 24 (4 from carbon, 6 from oxygen, and 14 from the two chlorine atoms). Next, we draw the skeletal structure, connecting the atoms with single bonds. The carbon atom is the central atom, bonded to one oxygen atom and two chlorine atoms.
We then distribute the remaining valence electrons around the atoms, ensuring that each atom has a full outer shell. The carbon atom has four valence electrons, the oxygen atom has six, and each chlorine atom has seven. By distributing the electrons, we find that the carbon atom has a double bond with the oxygen atom and single bonds with the two chlorine atoms. The resulting Lewis structure is:
C (central atom) = O (double bond) = Cl (single bond) - Cl (single bond)
This structure indicates that the carbon atom has a trigonal planar geometry, with bond angles of approximately 120 degrees. The double bond between the carbon and oxygen atoms is a result of the carbon atom's ability to form multiple bonds with oxygen.
Properties of COCl2
Phosgene is a colorless gas with a characteristic odor, often compared to moldy hay or green corn. It is highly toxic, with a threshold limit value (TLV) of 0.1 ppm in the workplace. Prolonged exposure to COCl2 can cause severe health effects, including respiratory problems, skin irritation, and eye damage.
The compound is also highly reactive, with a strong tendency to react with nucleophiles, such as water and ammonia. This reactivity is due to the electrophilic nature of the carbon atom, which is highly susceptible to nucleophilic attack.
| Property | Value |
|---|---|
| Molecular Weight | 98.92 g/mol |
| Boiling Point | 7.4°C |
| Melting Point | -127.8°C |
| Density | 1.4 g/cm³ |
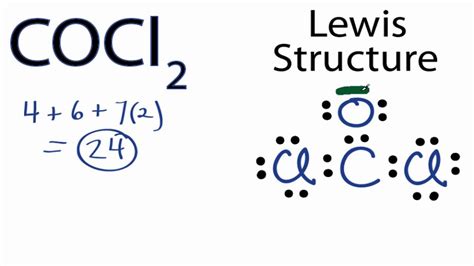
Synthesis and Applications of COCl2
Phosgene is synthesized through the reaction of carbon monoxide and chlorine gas. This reaction is highly exothermic, requiring careful control of temperature and pressure to avoid accidents.
COCl2 has various applications in the chemical industry, including the production of polyurethane foams, pesticides, and pharmaceuticals. The compound is also used as a reagent in organic synthesis, particularly in the production of isocyanates and carbamates.
Handling and Safety Precautions
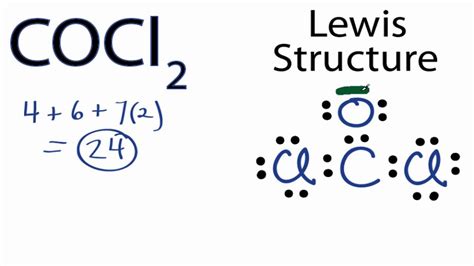
Due to the high toxicity and reactivity of COCl2, handling and storage of the compound require specialized equipment and safety precautions. Personnel handling phosgene must wear protective gear, including gloves, masks, and eye protection. The compound should be stored in a well-ventilated area, away from heat sources and flammable materials.
In the event of an accident or exposure, emergency responders should follow established protocols for handling toxic gas leaks. This includes evacuation of the area, use of personal protective equipment, and administration of first aid to affected individuals.
Environmental Impact of COCl2
The environmental impact of COCl2 is a significant concern due to its toxicity and persistence in the environment. Phosgene can contaminate soil, water, and air, posing a risk to human health and wildlife. The compound is also a potential contributor to climate change, with a global warming potential (GWP) of 0.6.
Efforts to minimize the environmental impact of COCl2 include the development of alternative synthesis methods, improved storage and handling procedures, and enhanced emergency response protocols. Regulatory agencies, such as the Environmental Protection Agency (EPA), play a crucial role in monitoring and controlling the use of phosgene in the chemical industry.
What is the primary use of phosgene in the chemical industry?
+The primary use of phosgene in the chemical industry is the production of polyurethane foams, pesticides, and pharmaceuticals.
What are the health effects of exposure to phosgene?
+Exposure to phosgene can cause severe health effects, including respiratory problems, skin irritation, and eye damage. Prolonged exposure can lead to more serious health issues, such as lung damage and cancer.
How is phosgene synthesized?
+Phosgene is synthesized through the reaction of carbon monoxide and chlorine gas. This reaction is highly exothermic, requiring careful control of temperature and pressure to avoid accidents.
In conclusion, the COCl2 Lewis structure is a fundamental concept in chemistry, essential for understanding the properties and reactivity of phosgene. The compound’s high toxicity and reactivity require specialized handling and storage procedures, as well as strict regulatory controls to minimize the risk of accidents and exposure. As the chemical industry continues to evolve, the development of alternative synthesis methods and improved safety protocols will be crucial in reducing the environmental impact of COCl2.