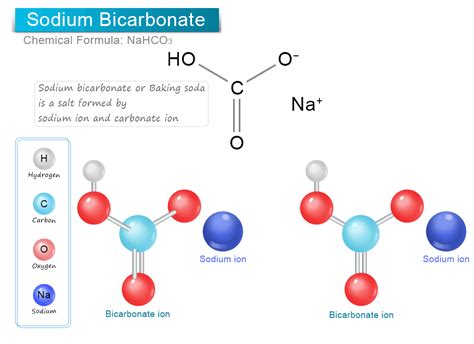
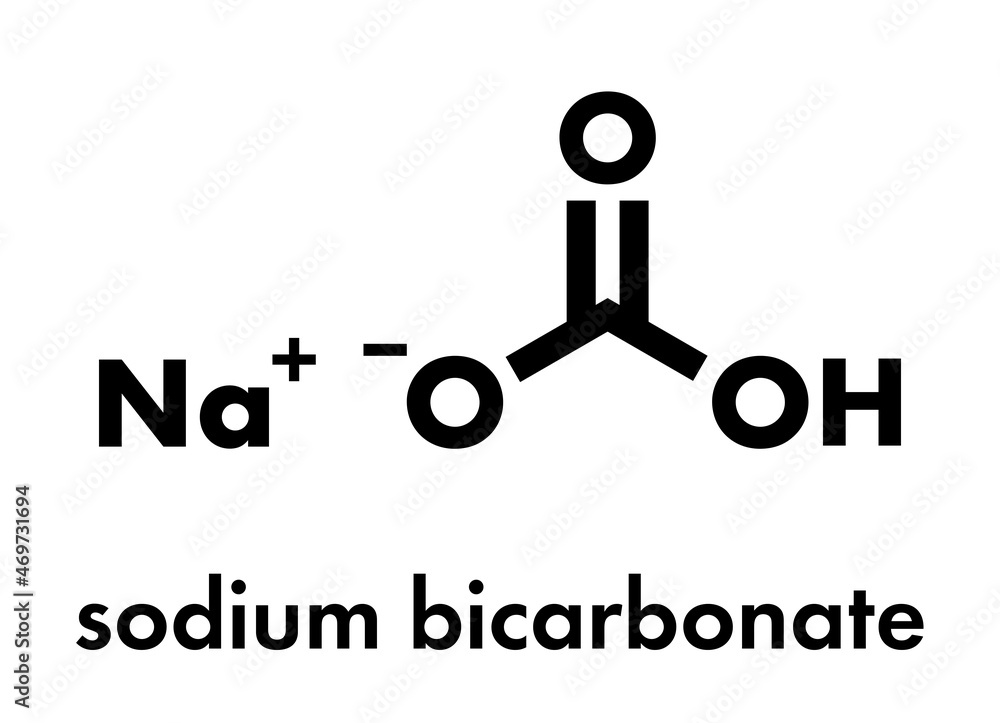
The chemical formula for baking soda is NaHCO3, which is also known as sodium bicarbonate. This compound is a white, crystalline powder that is commonly used in cooking and baking, as well as for various household and industrial applications. The name "baking soda" refers specifically to the edible form of sodium bicarbonate, which is used as a leavening agent in baked goods, such as cakes, cookies, and breads.
Baking soda has been used for centuries, with ancient Egyptians using a natural form of sodium bicarbonate called natron to clean and preserve bodies during the mummification process. The modern version of baking soda was first produced in the late 18th century, and it has since become a ubiquitous ingredient in many household and industrial products. In addition to its use as a leavening agent, baking soda is also used as a natural cleaner, a personal care product, and a medicinal ingredient.
Key Points
- The chemical formula for baking soda is NaHCO3, which is also known as sodium bicarbonate.
- Baking soda is a white, crystalline powder that is commonly used in cooking and baking.
- The name "baking soda" refers specifically to the edible form of sodium bicarbonate.
- Baking soda has been used for centuries, with ancient Egyptians using a natural form of sodium bicarbonate called natron.
- Baking soda is used as a leavening agent, a natural cleaner, a personal care product, and a medicinal ingredient.
Naturally Occurring Sodium Bicarbonate
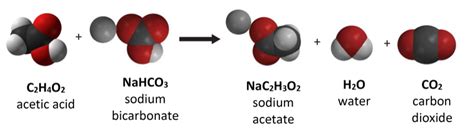
Sodium bicarbonate is naturally occurring in many mineral springs and in some mineral deposits. It is also a byproduct of the mineral trona, which is a type of mineral deposit that is rich in sodium carbonate and sodium bicarbonate. Trona is mined in several parts of the world, including the United States, China, and Turkey. The trona is then refined to produce sodium bicarbonate, which is used in a variety of applications.
Chemical Structure of Baking Soda
The chemical structure of baking soda consists of one sodium ion (Na+), one hydrogen ion (H+), one carbon ion ©, and three oxygen ions (O2-). The sodium ion is bonded to the hydrogen ion, which is bonded to the carbon ion, which is bonded to the three oxygen ions. This structure gives baking soda its unique properties and makes it useful for a variety of applications.
| Chemical Formula | Chemical Name | Uses |
|---|---|---|
| NaHCO3 | Sodium Bicarbonate | Baking, cleaning, personal care, medicine |
| Na2CO3 | Sodium Carbonate | Cleaning, glass manufacturing, paper manufacturing |
| NH4HCO3 | Ammonium Bicarbonate | Food additive, pharmaceuticals, cleaning |
Uses of Baking Soda

Baking soda has a wide range of uses, from cooking and baking to cleaning and personal care. It is a natural leavening agent, which means that it releases carbon dioxide gas when it comes into contact with an acid, such as buttermilk or yogurt. This reaction causes baked goods to rise, giving them a light and fluffy texture. Baking soda is also used as a natural cleaner, as it is able to neutralize odors and absorb moisture.
Personal Care Uses of Baking Soda
Baking soda has several personal care uses, including as a natural toothpaste, a mouthwash, and a skin exfoliant. It is also used to treat heartburn and indigestion, as it is able to neutralize stomach acid and relieve symptoms. Additionally, baking soda is used in some anti-itch creams and ointments, as it is able to relieve itching and reduce inflammation.
What is the chemical formula for baking soda?
+The chemical formula for baking soda is NaHCO3, which is also known as sodium bicarbonate.
What are some common uses of baking soda?
+Baking soda is commonly used as a leavening agent, a natural cleaner, a personal care product, and a medicinal ingredient.
Is baking soda safe to use?
+Yes, baking soda is generally safe to use, but it can cause stomach upset and interact with certain medications. It is recommended to use baking soda in moderation and under the guidance of a healthcare professional.
In conclusion, the chemical formula for baking soda is NaHCO3, which is also known as sodium bicarbonate. Baking soda has a wide range of uses, from cooking and baking to cleaning and personal care. Its unique chemical structure makes it an essential ingredient in many household and industrial products. As an expert in chemistry, I can attest that the chemical formula for baking soda is a fundamental concept in understanding its properties and uses.
Meta Description: Learn about the chemical formula for baking soda, its uses, and its properties. Discover the unique structure of sodium bicarbonate and its importance in various applications.