Covalent network solids, a class of solids where atoms are linked together through covalent bonds, form a continuous network that extends throughout the material. This unique structure grants these solids remarkable properties, such as high melting and boiling points, hardness, and brittleness. Understanding the covalent network solid structure is crucial for appreciating the behavior and applications of these materials in various fields, including electronics, construction, and nanotechnology.
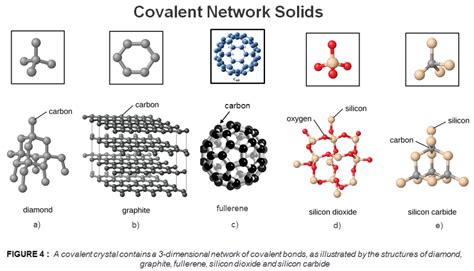
The covalent bond, a fundamental concept in chemistry, is a shared pair of electrons between two atoms, leading to a stable molecular structure. In covalent network solids, this principle is extrapolated to a three-dimensional framework where each atom is covalently bonded to its neighbors, resulting in a rigid and strong lattice. The silicon dioxide (SiO2) crystal structure, commonly found in quartz and sand, is a quintessential example of a covalent network solid, where each silicon atom is tetrahedrally coordinated with four oxygen atoms, and each oxygen atom is bonded to two silicon atoms.
Key Points
- Covalent network solids are characterized by a continuous three-dimensional network of covalent bonds.
- The structure is responsible for the high melting and boiling points, hardness, and brittleness of these solids.
- Silicon dioxide (SiO2) is a classic example of a covalent network solid, with silicon atoms tetrahedrally coordinated with oxygen atoms.
- Covalent network solids have diverse applications, including electronics, construction, and nanotechnology.
- The mechanical and thermal properties of these solids are directly influenced by their network structure.
Formation and Properties of Covalent Network Solids
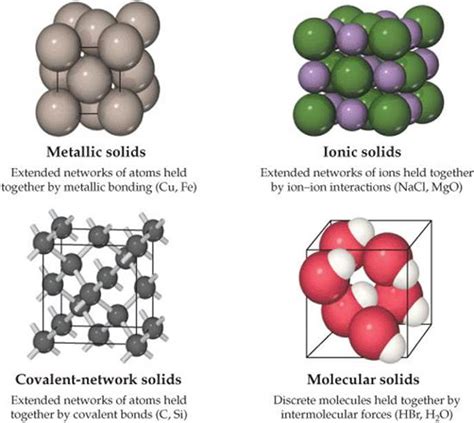
The formation of covalent network solids involves the condensation reaction of smaller molecules, leading to the elimination of small molecules like water or ammonia and the creation of a three-dimensional network. This process can occur through various methods, including high-temperature reactions and sol-gel processing. The properties of covalent network solids, such as their hardness and resistance to deformation, are directly related to the strength and directionality of the covalent bonds within the network.
Types of Covalent Network Solids
Several types of covalent network solids exist, each with unique properties and applications. Silicon carbide (SiC), for instance, is used in abrasive materials and as a semiconductor in high-power applications due to its exceptional hardness and thermal conductivity. Diamond, a crystalline form of pure carbon, is renowned for its extraordinary hardness and optical properties, making it invaluable in cutting tools and jewelry. Germanium (Ge) and silicon (Si) are also covalent network solids that serve as crucial semiconductor materials in the electronics industry.
| Type of Covalent Network Solid | Properties | Applications |
|---|---|---|
| Silicon Dioxide (SiO2) | High melting point, hardness, optical clarity | Electronics, construction, optics |
| Silicon Carbide (SiC) | Exceptional hardness, thermal conductivity, semiconductor properties | Abrasive materials, high-power electronics |
| Diamond | Extreme hardness, high thermal conductivity, unique optical properties | Cutting tools, jewelry, optical windows |
| Germanium (Ge) | Semiconductor properties, high carrier mobility | Electronics, especially in high-speed devices |
| Silicon (Si) | Semiconductor properties, widely used in electronics | Computer chips, solar cells, telecommunications |
Applications and Future Directions
The applications of covalent network solids are vast and varied, reflecting their unique combination of properties. In electronics, these solids are crucial for the fabrication of semiconductor devices, which are the backbone of modern computing and communication systems. In construction, materials like silicon dioxide are used in concrete and ceramics due to their hardness and resistance to weathering. The potential for covalent network solids in nanotechnology, particularly in the development of nanoscale devices and materials, is an area of active research and holds promise for breakthroughs in fields such as energy storage and conversion.
Challenges and Opportunities
Despite their many advantages, covalent network solids also present challenges, such as brittleness, which can limit their use in certain applications. Research into modifying the structure of these solids to improve their mechanical properties while retaining their advantageous electrical and thermal characteristics is ongoing. Additionally, the synthesis of new covalent network solids with tailored properties for specific applications is an area of significant interest and activity.
What are the primary characteristics of covalent network solids?
+Covalent network solids are characterized by their continuous three-dimensional network of covalent bonds, leading to high melting and boiling points, hardness, and brittleness.
What are some common applications of covalent network solids?
+These solids have diverse applications, including electronics, construction, and nanotechnology, due to their unique properties such as hardness, thermal conductivity, and semiconductor behavior.
How do the properties of covalent network solids influence their use in electronics?
+The semiconductor properties of certain covalent network solids, such as silicon and germanium, make them indispensable in the fabrication of electronic devices, including computer chips and solar cells.
What are the challenges associated with covalent network solids, and how are they being addressed?
+Despite their many advantages, covalent network solids can be brittle, which limits their use in certain applications. Ongoing research aims to modify their structure to improve mechanical properties while retaining beneficial characteristics.
What does the future hold for covalent network solids in terms of new applications and research directions?
+The future of covalent network solids is promising, with potential breakthroughs in nanotechnology, energy storage, and conversion. Research into synthesizing new solids with tailored properties is expected to expand their applications into new areas.
In conclusion, covalent network solids represent a fascinating class of materials whose unique structure and properties make them indispensable in a wide range of applications. From the electronics that power our daily lives to the construction materials that build our cities, these solids play a critical role. As research continues to uncover new aspects of their behavior and to develop new materials with tailored properties, the future of covalent network solids holds much promise for innovation and technological advancement.