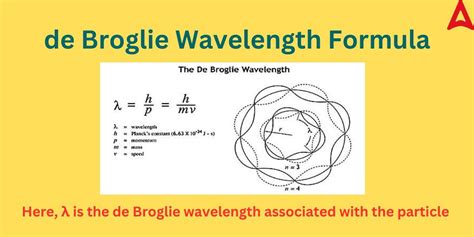
The concept of de Broglie wavelength, introduced by Louis de Broglie in 1924, revolutionized the field of quantum mechanics by suggesting that particles, such as electrons, exhibit wave-like behavior. This idea, which seemed radical at the time, has since been extensively validated through numerous experiments and has become a cornerstone of modern physics. The de Broglie hypothesis states that any moving particle has a wave associated with it, and the wavelength of this wave (λ) is inversely proportional to the particle's momentum (p = mv, where m is the mass of the particle and v is its velocity). The relationship is given by the equation λ = h / p, where h is Planck's constant.
This concept has profound implications for our understanding of matter and energy at the atomic and subatomic level. It not only explains the behavior of electrons in atoms but also underpins many phenomena observed in particle physics. For instance, the wave nature of particles helps explain the diffraction patterns observed in electron diffraction experiments, similar to those seen in light diffraction, further solidifying the wave-particle duality principle. Moreover, the de Broglie wavelength plays a critical role in the design and operation of electron microscopes, which can achieve higher resolutions than light microscopes due to the shorter wavelengths of electrons compared to visible light.
Key Points
- The de Broglie wavelength of a particle is inversely proportional to its momentum, given by λ = h / p.
- This concept is fundamental to quantum mechanics and explains the wave-like behavior of particles.
- The de Broglie hypothesis has been experimentally confirmed through electron diffraction experiments and other means.
- It underpins the operation of electron microscopes and other technologies that rely on the wave nature of particles.
- The concept of wave-particle duality, which includes the de Broglie wavelength, is crucial for understanding phenomena at the atomic and subatomic level.
Derivation and Significance of de Broglie Wavelength
The derivation of the de Broglie wavelength equation starts with the assumption that particles, such as electrons, can exhibit wave-like properties. By combining the principles of quantum mechanics and special relativity, de Broglie proposed that the wavelength of a particle could be related to its momentum. This relationship, λ = h / p, where λ is the wavelength of the particle, h is Planck’s constant (approximately 6.626 x 10^-34 J s), and p is the momentum of the particle, provided a theoretical framework for understanding the behavior of particles at the quantum level.
Experimental Verification
The de Broglie hypothesis was experimentally verified through several experiments, most notably the electron diffraction experiments. In these experiments, a beam of electrons is passed through a crystal, and the resulting diffraction pattern is observed. The pattern shows that electrons, like light, can diffract around the atoms in the crystal, creating an interference pattern that is characteristic of wave behavior. This experiment, first performed by Clinton Davisson and Lester Germer in 1927, provided strong evidence for the wave nature of particles and confirmed the de Broglie hypothesis.
| Particle | Mass (kg) | Velocity (m/s) | Momentum (kg m/s) | de Broglie Wavelength (m) |
|---|---|---|---|---|
| Electron | 9.109 x 10^-31 | 100 | 9.109 x 10^-29 | 7.274 x 10^-10 |
| Proton | 1.672 x 10^-27 | 100 | 1.672 x 10^-25 | 3.971 x 10^-12 |
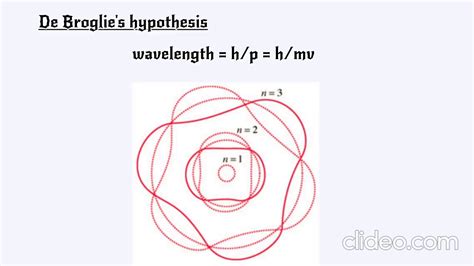
Applications and Implications

The concept of de Broglie wavelength has numerous applications in physics and engineering. One of the most significant applications is in the field of electron microscopy. Electron microscopes use a beam of electrons to “illuminate” the specimen and produce an image. The wavelength of the electrons determines the resolution of the microscope, with shorter wavelengths allowing for higher resolutions. The de Broglie wavelength equation is used to calculate the wavelength of the electrons based on their energy, which is critical for achieving high-resolution images.
Quantum Computing and Nanotechnology
The de Broglie wavelength also plays a role in the development of quantum computing and nanotechnology. In these fields, understanding the behavior of particles at the quantum level is essential for designing and operating devices. The wave nature of particles, as described by the de Broglie hypothesis, is crucial for phenomena such as quantum tunneling and interference, which are exploited in quantum computing and nanoscale devices.
In conclusion, the de Broglie wavelength is a fundamental concept in quantum mechanics that has been extensively verified through experiments. Its implications are far-reaching, influencing our understanding of matter and energy at the atomic and subatomic level, and underpinning the development of advanced technologies such as electron microscopes, quantum computers, and nanoscale devices. The study of de Broglie wavelength continues to be an active area of research, with ongoing efforts to explore its applications and implications in various fields of physics and engineering.
What is the significance of the de Broglie wavelength in physics?
+The de Broglie wavelength is significant because it explains the wave-like behavior of particles, such as electrons, and has been experimentally verified. It underpins many phenomena in quantum mechanics and is crucial for the operation of electron microscopes and other technologies.
How is the de Broglie wavelength calculated?
+The de Broglie wavelength is calculated using the equation λ = h / p, where λ is the wavelength of the particle, h is Planck’s constant, and p is the momentum of the particle.
What are some applications of the de Broglie wavelength?
+Some applications of the de Broglie wavelength include electron microscopy, quantum computing, and nanotechnology. Understanding the wave nature of particles is crucial for designing and operating devices in these fields.