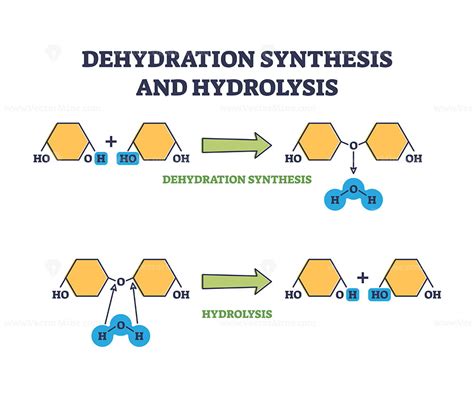
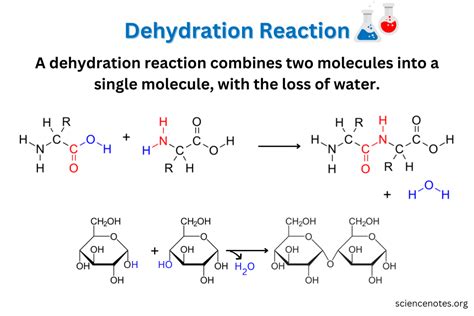
Dehydration synthesis is a fundamental concept in biochemistry and organic chemistry, referring to a type of chemical reaction where two molecules combine to form a new compound, releasing water (H2O) in the process. This reaction is pivotal in the formation of various biological molecules, including carbohydrates, proteins, and nucleic acids. The process involves the condensation of two molecules, resulting in the loss of a hydroxyl group (-OH) from one molecule and a hydrogen atom (H) from the other, forming a new covalent bond between the two molecules.
The importance of dehydration synthesis cannot be overstated, as it plays a critical role in the biosynthesis of complex molecules within living organisms. For instance, in the context of carbohydrate metabolism, dehydration synthesis is essential for the formation of glycogen, a complex carbohydrate stored in the liver and muscles. Similarly, in protein synthesis, dehydration synthesis is involved in the formation of peptide bonds between amino acids, giving rise to the primary structure of proteins.
Key Points
- Dehydration synthesis involves the combination of two molecules with the release of water.
- This reaction is crucial for the biosynthesis of carbohydrates, proteins, and nucleic acids.
- The process involves the formation of a new covalent bond between the two molecules.
- Dehydration synthesis plays a key role in the formation of glycogen and the synthesis of proteins.
- This reaction type is fundamental in understanding biochemical pathways and metabolic processes.
Mechanism of Dehydration Synthesis
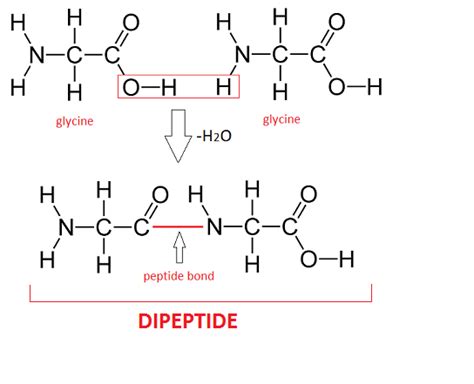
The mechanism of dehydration synthesis involves several steps, starting with the alignment of the two reactant molecules in a manner that facilitates the formation of a new covalent bond. This is often mediated by enzymes, which provide a catalytic environment that lowers the activation energy required for the reaction to proceed. The reaction typically involves the nucleophilic attack of one molecule on the other, leading to the formation of a transition state that eventually results in the release of water and the formation of the product.
Dehydration synthesis reactions are often reversible, with the reverse reaction being known as hydrolysis. Hydrolysis involves the cleavage of a molecule using water, resulting in the breakage of a covalent bond and the addition of a hydroxyl group to one of the products and a hydrogen atom to the other. The reversibility of dehydration synthesis and hydrolysis reactions is a fundamental aspect of biochemical pathways, allowing for the dynamic regulation of metabolic processes in response to cellular needs.
Types of Dehydration Synthesis
There are several types of dehydration synthesis reactions, each characterized by the specific molecules involved and the nature of the bond formed. For example, in the context of carbohydrate chemistry, dehydration synthesis is used to form glycosidic bonds between sugars. In protein chemistry, the reaction is used to form peptide bonds between amino acids. Additionally, dehydration synthesis is involved in the formation of phosphodiester bonds in nucleic acids, which link nucleotides together in DNA and RNA.
| Type of Bond | Example |
|---|---|
| Glycosidic bond | Formation of disaccharides from monosaccharides |
| Peptide bond | Formation of proteins from amino acids |
| Phosphodiester bond | Formation of nucleic acids from nucleotides |
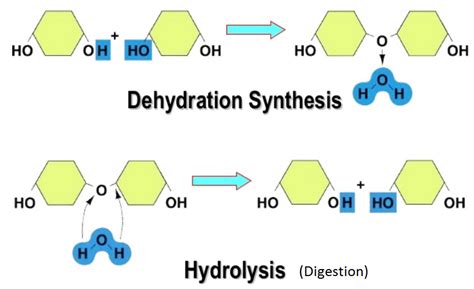
Biological Significance of Dehydration Synthesis
The biological significance of dehydration synthesis extends beyond the formation of complex biomolecules. It plays a critical role in various cellular processes, including energy storage and release, cell signaling, and the regulation of metabolic pathways. For instance, the formation of glycogen through dehydration synthesis reactions provides a means for cells to store energy in a compact form, which can be rapidly mobilized when needed.
Moreover, dehydration synthesis reactions are tightly regulated within cells, with enzymes and other regulatory molecules controlling the rates of these reactions. This regulation allows cells to respond to changes in their environment and to maintain homeostasis, which is essential for survival. The dysregulation of dehydration synthesis reactions has been implicated in various diseases, highlighting the importance of understanding these processes at a molecular level.
Regulation of Dehydration Synthesis
The regulation of dehydration synthesis involves a complex interplay of enzymes, hormones, and other signaling molecules. Enzymes that catalyze dehydration synthesis reactions are subject to allosteric control, where the binding of regulatory molecules can either increase or decrease the enzyme’s activity. Additionally, the expression of genes encoding these enzymes can be regulated at the transcriptional level, allowing for long-term adjustments in the rates of dehydration synthesis reactions.
Hormones also play a crucial role in regulating dehydration synthesis, particularly in the context of energy metabolism. For example, insulin and glucagon regulate the formation and breakdown of glycogen in the liver and muscles, ensuring that blood glucose levels remain within a narrow range. The regulation of dehydration synthesis reactions by hormones and other signaling molecules underscores the integrated nature of cellular metabolism and the importance of these reactions in maintaining cellular homeostasis.
What is the primary outcome of a dehydration synthesis reaction?
+The primary outcome of a dehydration synthesis reaction is the formation of a new covalent bond between two molecules, accompanied by the release of water.
What types of biological molecules are formed through dehydration synthesis?
+Dehydration synthesis is involved in the formation of carbohydrates, proteins, and nucleic acids, which are essential for various cellular processes.
How is dehydration synthesis regulated within cells?
+Dehydration synthesis is regulated through the action of enzymes, hormones, and other signaling molecules, which control the rates of these reactions and ensure that they are coordinated with cellular needs.
In conclusion, dehydration synthesis is a vital biochemical reaction that underpins the formation of complex biological molecules. Its significance extends beyond the molecular level, influencing various cellular processes and contributing to the overall health and function of organisms. By understanding the mechanisms, regulation, and biological significance of dehydration synthesis, scientists can gain insights into the intricate workings of cellular metabolism and the dynamic interplay between different biochemical pathways.