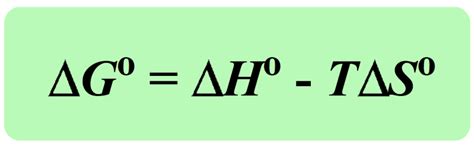The Delta G formula, also known as the Gibbs free energy equation, is a fundamental concept in thermodynamics that helps predict the spontaneity of a chemical reaction. The formula is expressed as ΔG = ΔH - TΔS, where ΔG is the change in Gibbs free energy, ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy. Understanding the Delta G formula is crucial in various fields, including chemistry, biology, and physics. In this article, we will explore five ways to apply the Delta G formula in different contexts.
Key Points
- The Delta G formula is used to predict the spontaneity of a chemical reaction.
- ΔG is related to the change in enthalpy (ΔH) and entropy (ΔS) of a system.
- The temperature (T) plays a crucial role in determining the spontaneity of a reaction.
- The Delta G formula has applications in various fields, including chemistry, biology, and physics.
- Understanding the Delta G formula is essential for predicting the feasibility of a chemical reaction.
Understanding the Delta G Formula

The Delta G formula is a mathematical expression that combines the changes in enthalpy and entropy of a system to predict the spontaneity of a chemical reaction. A negative ΔG value indicates a spontaneous reaction, while a positive ΔG value indicates a non-spontaneous reaction. The formula is often used to determine the feasibility of a reaction and to predict the direction of a reaction.
Applications of the Delta G Formula
The Delta G formula has numerous applications in various fields. In chemistry, it is used to predict the spontaneity of chemical reactions and to determine the feasibility of a reaction. In biology, it is used to understand the energy changes that occur during metabolic processes. In physics, it is used to study the thermodynamic properties of materials.
| Field of Study | Application of Delta G Formula |
|---|---|
| Chemistry | Predicting the spontaneity of chemical reactions |
| Biology | Understanding the energy changes during metabolic processes |
| Physics | Studying the thermodynamic properties of materials |
5 Ways to Apply the Delta G Formula
The Delta G formula can be applied in various ways to understand the thermodynamics of a system. Here are five ways to apply the Delta G formula:
1. Predicting the Spontaneity of a Reaction
The Delta G formula can be used to predict the spontaneity of a chemical reaction. By calculating the ΔG value, we can determine whether a reaction is spontaneous or non-spontaneous. A negative ΔG value indicates a spontaneous reaction, while a positive ΔG value indicates a non-spontaneous reaction.
2. Determining the Feasibility of a Reaction
The Delta G formula can be used to determine the feasibility of a chemical reaction. By calculating the ΔG value, we can predict whether a reaction is feasible or not. A reaction with a negative ΔG value is feasible, while a reaction with a positive ΔG value is not feasible.
3. Understanding the Effect of Temperature on a Reaction
The Delta G formula can be used to understand the effect of temperature on a chemical reaction. By calculating the ΔG value at different temperatures, we can determine how the spontaneity of a reaction changes with temperature.
4. Calculating the Equilibrium Constant of a Reaction
The Delta G formula can be used to calculate the equilibrium constant of a chemical reaction. By calculating the ΔG value, we can determine the equilibrium constant of a reaction, which is a measure of the ratio of the concentrations of the products to the reactants at equilibrium.
5. Understanding the Energy Changes During a Reaction
The Delta G formula can be used to understand the energy changes that occur during a chemical reaction. By calculating the ΔG value, we can determine the energy changes that occur during a reaction, including the change in enthalpy and entropy.
What is the Delta G formula used for?
+The Delta G formula is used to predict the spontaneity of a chemical reaction and to determine the feasibility of a reaction.
How does the Delta G formula relate to the change in enthalpy and entropy?
+The Delta G formula combines the changes in enthalpy and entropy of a system to predict the spontaneity of a chemical reaction.
What is the significance of the temperature in the Delta G formula?
+The temperature plays a crucial role in determining the spontaneity of a reaction, as it affects the change in Gibbs free energy.