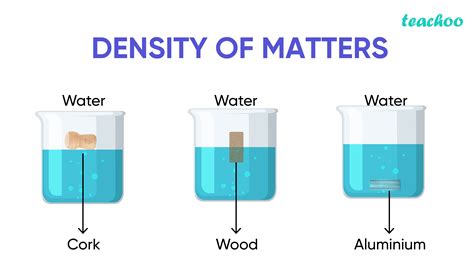
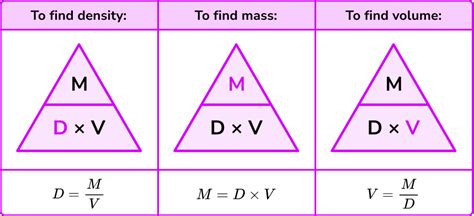
Density is a fundamental concept in physics that describes the amount of mass contained in a given volume of a substance. It is defined as the ratio of mass to volume, and is typically denoted by the symbol ρ (rho). The formula for density is ρ = m/V, where m is the mass of the substance and V is its volume. Density is an important property of a substance because it can be used to identify the substance, determine its buoyancy, and calculate its weight.
Understanding Density

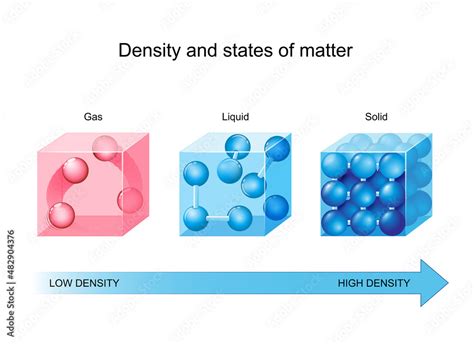
Density is a measure of how tightly packed the molecules of a substance are. Substances with high densities have molecules that are closely packed, while substances with low densities have molecules that are more spread out. For example, lead has a high density of 11.34 g/cm³, which means that it is very heavy for its size. On the other hand, air has a low density of approximately 0.0012 g/cm³, which is why it is so light.
Types of Density
There are several types of density, including:
- True density: the density of a substance in its pure form, without any air pockets or impurities.
- Bulk density: the density of a substance in a powdered or granular form, including any air pockets or voids.
- Apparent density: the density of a substance as it appears to be, including any air pockets or impurities.
Understanding the different types of density is important in various fields, such as engineering, chemistry, and physics. For example, in construction, the bulk density of a material is important because it affects the material's strength and durability.
| Substance | Density (g/cm³) |
|---|---|
| Lead | 11.34 |
| Copper | 8.96 |
| Water | 1.00 |
| Air | 0.0012 |
Key Points
- Density is defined as the ratio of mass to volume.
- Density is an important property of a substance that can be used to identify it and determine its buoyancy.
- There are several types of density, including true density, bulk density, and apparent density.
- Understanding density is crucial in many real-world applications, such as materials science and chemistry.
- Density is typically denoted by the symbol ρ (rho) and is measured in units of g/cm³ or kg/m³.
Calculating Density
Calculating density is a straightforward process that involves measuring the mass and volume of a substance. The formula for density is ρ = m/V, where m is the mass of the substance and V is its volume. For example, if you have a cube of lead that measures 10 cm on each side, and it has a mass of 1000 grams, you can calculate its density as follows:
ρ = m/V = 1000 g / (10 cm x 10 cm x 10 cm) = 1000 g / 1000 cm³ = 1 g/cm³
However, this is not the true density of lead, which is actually 11.34 g/cm³. This is because the cube of lead is not a perfect cube, and it may have air pockets or impurities that affect its density.
Factors that Affect Density
There are several factors that can affect the density of a substance, including:
- Temperature: density typically decreases with increasing temperature.
- Pressure: density typically increases with increasing pressure.
- Purity: density can be affected by the presence of impurities or air pockets.
Understanding these factors is important because they can affect the accuracy of density measurements. For example, if you are measuring the density of a substance at high temperatures, you may need to take into account the expansion of the substance and the resulting decrease in density.
What is the difference between true density and bulk density?
+True density is the density of a substance in its pure form, without any air pockets or impurities. Bulk density, on the other hand, is the density of a substance in a powdered or granular form, including any air pockets or voids.
How do you calculate density?
+Density is calculated by dividing the mass of a substance by its volume. The formula for density is ρ = m/V, where m is the mass of the substance and V is its volume.
What are some common units of density?
+Some common units of density include g/cm³, kg/m³, and lb/ft³.
Meta description: Learn about density, including its definition, types, and calculation. Understand how density is affected by temperature, pressure, and purity, and how it is used in real-world applications. (147 characters)