Lead, a chemical element with the symbol Pb, has been a crucial component in various industries for centuries. Its unique properties make it an ideal material for a wide range of applications, from batteries and ammunition to radiation shielding and pigments. Despite its importance, lead is also known for its toxicity, posing significant health risks to humans and the environment. In this article, we will delve into the world of lead, exploring its history, properties, uses, and environmental impact.
History and Discovery of Lead

Lead has been used by humans for thousands of years, with evidence of its use dating back to ancient civilizations in Egypt, Greece, and Rome. The element was highly valued for its malleability, ductility, and resistance to corrosion, making it an ideal material for crafting tools, weapons, and other objects. The Romans, in particular, were known for their extensive use of lead in plumbing, which is where the word “plumber” originates. Over time, the use of lead has evolved, with new technologies and applications emerging. Today, lead is a critical component in the production of batteries, electronics, and other modern technologies.
Key Points
- Lead has been used by humans for thousands of years, with evidence of its use dating back to ancient civilizations.
- The element is highly valued for its malleability, ductility, and resistance to corrosion.
- Lead is a critical component in the production of batteries, electronics, and other modern technologies.
- The use of lead poses significant health risks to humans and the environment due to its toxicity.
- Efforts are being made to reduce the environmental impact of lead, including the development of lead-free alternatives and more efficient recycling processes.
Properties and Characteristics of Lead
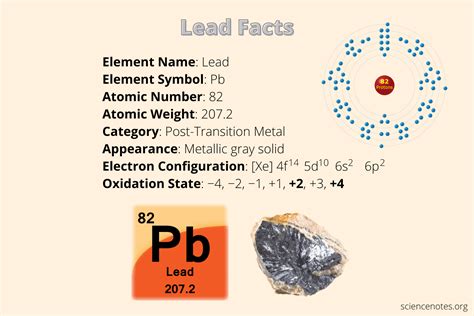
Lead is a post-transition metal with a bluish-white color and a bright luster. It has a melting point of 327.5°C and a boiling point of 1749°C, making it a relatively soft and fusible metal. Lead is also highly malleable and ductile, allowing it to be easily shaped and molded into various forms. One of the most distinctive properties of lead is its high density, which makes it an ideal material for radiation shielding and other applications where weight and stability are critical. However, lead’s toxicity is a significant concern, as it can cause a range of health problems, including neurological damage, organ failure, and even death.
| Property | Value |
|---|---|
| Melting Point | 327.5°C |
| Boiling Point | 1749°C |
| Density | 11.34 g/cm³ |
| Atomic Number | 82 |
| Atomic Mass | 207.2 u |
Uses and Applications of Lead
Despite the risks associated with lead, it remains a critical component in various industries. The most significant use of lead is in the production of lead-acid batteries, which are used to power vehicles, backup power systems, and other applications. Lead is also used in the manufacture of electronics, including semiconductors, transistors, and diodes. Additionally, lead is used in radiation shielding, due to its high density and ability to absorb radiation. Other applications of lead include pigments, ammunition, and solders.
Environmental Impact of Lead
The use of lead poses significant environmental risks, as it can contaminate soil, water, and air. Lead pollution can occur through various pathways, including industrial processes, waste disposal, and vehicle emissions. Exposure to lead can cause a range of health problems, including neurological damage, organ failure, and even death. Efforts are being made to reduce the environmental impact of lead, including the development of lead-free alternatives, more efficient recycling processes, and stricter regulations on lead emissions.
What are the main uses of lead?
+The main uses of lead include the production of lead-acid batteries, electronics, radiation shielding, pigments, ammunition, and solders.
What are the health risks associated with lead exposure?
+Exposure to lead can cause a range of health problems, including neurological damage, organ failure, and even death. Children and pregnant women are particularly vulnerable to the effects of lead poisoning.
What is being done to reduce the environmental impact of lead?
+Efforts are being made to reduce the environmental impact of lead, including the development of lead-free alternatives, more efficient recycling processes, and stricter regulations on lead emissions. Additionally, education and awareness campaigns are being implemented to inform the public about the risks associated with lead exposure.
In conclusion, lead is a complex and multifaceted element with a rich history, unique properties, and diverse applications. While it poses significant health and environmental risks, efforts are being made to minimize its use and promote safer alternatives. As we move forward, it’s essential to continue exploring new technologies and strategies to reduce the impact of lead and create a safer, more sustainable future for all.