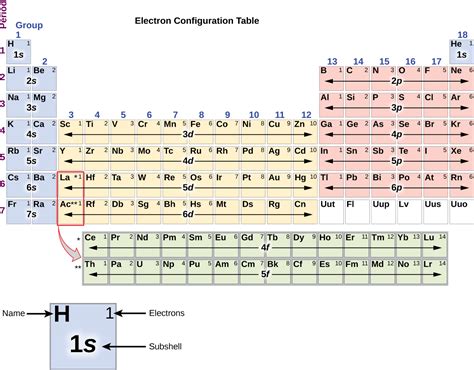
The electron configuration table is a fundamental tool in chemistry, used to predict the behavior of atoms and molecules. It is a tabular representation of the arrangement of electrons in an atom, which is essential for understanding the chemical properties and reactivity of elements. In this article, we will provide a comprehensive guide to the electron configuration table, including its history, structure, and applications.
Key Points
- The electron configuration table is a tabular representation of the arrangement of electrons in an atom.
- The table is divided into rows and columns, with each row representing a principal energy level and each column representing a subshell.
- The electron configuration of an atom is written in a shorthand notation, with the number of electrons in each subshell indicated by a superscript number.
- The electron configuration table is used to predict the chemical properties and reactivity of elements.
- The table is essential for understanding the behavior of atoms and molecules in chemical reactions.
History of the Electron Configuration Table
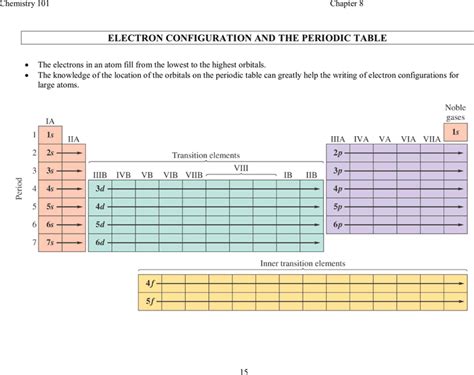
The electron configuration table has its roots in the early 20th century, when the concept of atomic structure was first developed. The table was initially based on the Bohr model of the atom, which described the arrangement of electrons in energy levels or shells around the nucleus. Over time, the table has undergone significant revisions and refinements, incorporating new discoveries and theories in atomic physics. Today, the electron configuration table is a widely accepted and indispensable tool in chemistry, used to predict the behavior of atoms and molecules.
Structure of the Electron Configuration Table
The electron configuration table is divided into rows and columns, with each row representing a principal energy level and each column representing a subshell. The table is typically arranged in a hierarchical manner, with the lowest energy levels at the top and the highest energy levels at the bottom. Each subshell is designated by a letter (s, p, d, f), which indicates the orbital shape and orientation. The number of electrons in each subshell is indicated by a superscript number, which is used to write the electron configuration of an atom in shorthand notation.
| Principal Energy Level | Subshell | Number of Electrons |
|---|---|---|
| 1 | 1s | 2 |
| 2 | 2s | 2 |
| 2 | 2p | 6 |
| 3 | 3s | 2 |
| 3 | 3p | 6 |
| 3 | 3d | 10 |

Applications of the Electron Configuration Table

The electron configuration table has numerous applications in chemistry, including predicting the chemical properties and reactivity of elements. The table is used to determine the electron configuration of an atom, which is essential for understanding the behavior of atoms and molecules in chemical reactions. The table is also used to predict the formation of compounds, including ionic and covalent bonds. Additionally, the table is used to explain the periodic trends in the periodic table, including the variation in atomic radius, electronegativity, and ionization energy.
Predicting Chemical Properties and Reactivity
The electron configuration table is used to predict the chemical properties and reactivity of elements by determining the number of valence electrons in an atom. Valence electrons are the electrons in the outermost energy level of an atom, which are involved in chemical bonding. The number of valence electrons in an atom determines its chemical reactivity, with atoms having a full outer energy level being less reactive than atoms with a partially filled outer energy level.
What is the electron configuration table used for?
+The electron configuration table is used to predict the chemical properties and reactivity of elements, including the formation of compounds and the behavior of atoms and molecules in chemical reactions.
How is the electron configuration of an atom written in shorthand notation?
+The electron configuration of an atom is written in shorthand notation by indicating the number of electrons in each subshell with a superscript number. For example, the electron configuration of carbon is 1s² 2s² 2p².
What is the importance of the electron configuration table in chemistry?
+The electron configuration table is essential for understanding the behavior of atoms and molecules in chemical reactions. It provides a framework for predicting the chemical properties and reactivity of elements, which is critical for understanding chemical reactions and the formation of compounds.
In conclusion, the electron configuration table is a fundamental tool in chemistry, used to predict the behavior of atoms and molecules. The table is divided into rows and columns, with each row representing a principal energy level and each column representing a subshell. The electron configuration of an atom is written in shorthand notation, with the number of electrons in each subshell indicated by a superscript number. The table has numerous applications in chemistry, including predicting the chemical properties and reactivity of elements, and is essential for understanding the behavior of atoms and molecules in chemical reactions.
Meta Description: Learn about the electron configuration table, a fundamental tool in chemistry used to predict the behavior of atoms and molecules. Understand the structure and applications of the table, and how it is used to predict chemical properties and reactivity. (149 characters)