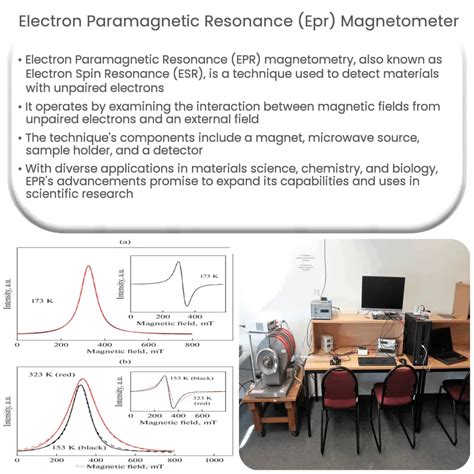
Electron Paramagnetic Resonance (EPR) is a highly versatile and sensitive spectroscopic technique that has been widely used in various fields, including physics, chemistry, biology, and materials science. At its core, EPR is a method that detects the absorption of microwave radiation by paramagnetic substances, which are materials that contain unpaired electrons. These unpaired electrons are responsible for the unique properties of paramagnetic materials and play a crucial role in many biological and chemical processes.
The principle of EPR is based on the Zeeman effect, which describes the splitting of energy levels in the presence of an external magnetic field. In the case of EPR, the energy levels of the unpaired electrons are split into two states, one with the electron spin aligned with the magnetic field (ms = +1/2) and the other with the electron spin opposed to the magnetic field (ms = -1/2). When a sample containing unpaired electrons is placed in a magnetic field and irradiated with microwave radiation, the electrons can absorb energy and transition from one state to the other, resulting in a characteristic resonance signal.
Key Points
- EPR is a spectroscopic technique that detects the absorption of microwave radiation by paramagnetic substances.
- The technique is based on the Zeeman effect, which describes the splitting of energy levels in the presence of an external magnetic field.
- EPR has a wide range of applications, including the study of free radicals, transition metal ions, and biological systems.
- The technique provides valuable information about the structure and dynamics of paramagnetic substances.
- EPR can be used to study the properties of materials at the molecular level, making it a powerful tool for materials scientists and chemists.
Principle of EPR

The principle of EPR can be understood by considering the behavior of an isolated electron in a magnetic field. The electron has a spin of 1⁄2 and a magnetic moment associated with it. When the electron is placed in a magnetic field, its energy levels are split into two states, one with the electron spin aligned with the magnetic field (ms = +1⁄2) and the other with the electron spin opposed to the magnetic field (ms = -1⁄2). The energy difference between these two states is given by the Zeeman equation, ΔE = gβH, where g is the gyromagnetic ratio, β is the Bohr magneton, and H is the magnetic field strength.
EPR Spectroscopy
EPR spectroscopy is a technique that measures the absorption of microwave radiation by paramagnetic substances. The technique involves placing a sample in a magnetic field and irradiating it with microwave radiation. The microwave radiation is tuned to a specific frequency, and the magnetic field is swept through a range of values. When the energy difference between the two states of the unpaired electron matches the energy of the microwave radiation, the electron absorbs energy and transitions from one state to the other, resulting in a characteristic resonance signal.
| Category | Description |
|---|---|
| EPR Spectroscopy | Measures the absorption of microwave radiation by paramagnetic substances. |
| Magnetic Field | Split the energy levels of the unpaired electrons into two states. |
| Microwave Radiation | Causes the electrons to transition from one state to the other, resulting in a resonance signal. |
Applications of EPR

EPR has a wide range of applications in various fields, including physics, chemistry, biology, and materials science. Some of the key applications of EPR include the study of free radicals, transition metal ions, and biological systems. EPR can provide valuable information about the structure and dynamics of paramagnetic substances, making it a powerful tool for understanding the properties of materials at the molecular level.
Study of Free Radicals
Free radicals are highly reactive molecules that contain unpaired electrons. EPR is a powerful tool for studying the properties of free radicals, as it can provide information about their structure and dynamics. Free radicals play a crucial role in many biological and chemical processes, including oxidation reactions and cell signaling pathways.
Study of Transition Metal Ions
Transition metal ions are paramagnetic substances that contain unpaired electrons. EPR can provide valuable information about the structure and dynamics of transition metal ions, making it a powerful tool for understanding their properties and behavior. Transition metal ions play a crucial role in many biological and chemical processes, including enzyme catalysis and electron transfer reactions.
What is the principle of EPR?
+The principle of EPR is based on the Zeeman effect, which describes the splitting of energy levels in the presence of an external magnetic field.
What are the applications of EPR?
+EPR has a wide range of applications in various fields, including physics, chemistry, biology, and materials science. Some of the key applications of EPR include the study of free radicals, transition metal ions, and biological systems.
What information can EPR provide about paramagnetic substances?
+EPR can provide valuable information about the structure and dynamics of paramagnetic substances, including their spin state, magnetic moment, and relaxation times.
In conclusion, EPR is a highly versatile and sensitive spectroscopic technique that has been widely used in various fields, including physics, chemistry, biology, and materials science. The technique is based on the Zeeman effect, which describes the splitting of energy levels in the presence of an external magnetic field. EPR can provide valuable information about the structure and dynamics of paramagnetic substances, making it a powerful tool for understanding the properties of materials at the molecular level.