The Emory University Institutional Review Board (IRB) guidelines are a set of regulations and procedures designed to ensure the protection of human subjects involved in research studies. As a prestigious institution, Emory University is committed to upholding the highest standards of ethical conduct in research, and the IRB plays a crucial role in achieving this goal. The IRB guidelines are based on federal regulations, state laws, and institutional policies, and are designed to provide a framework for researchers to conduct studies that are both scientifically valid and ethically sound.
Overview of Emory University IRB Guidelines

The Emory University IRB guidelines cover a wide range of topics, including the principles of informed consent, the protection of vulnerable populations, and the conduct of research involving human subjects. The guidelines are divided into several sections, each addressing a specific aspect of research involving human subjects. These sections include the definition of human subjects research, the requirements for informed consent, the procedures for obtaining IRB approval, and the responsibilities of researchers and IRB members. By following these guidelines, researchers can ensure that their studies are conducted in a manner that respects the rights and welfare of human subjects.
Key Principles of Emory University IRB Guidelines
The Emory University IRB guidelines are based on several key principles, including respect for persons, beneficence, non-maleficence, and justice. Respect for persons requires that researchers obtain informed consent from human subjects before enrolling them in a study. Beneficence requires that researchers maximize the benefits of their studies and minimize the risks to human subjects. Non-maleficence requires that researchers do no harm to human subjects, and justice requires that researchers ensure that the benefits and risks of their studies are distributed fairly. By following these principles, researchers can ensure that their studies are conducted in a manner that is consistent with the highest ethical standards.
| Category | Description |
|---|---|
| Respect for Persons | Obtaining informed consent from human subjects |
| Beneficence | Maximizing benefits and minimizing risks to human subjects |
| Non-Maleficence | Doing no harm to human subjects |
| Justice | Ensuring fair distribution of benefits and risks |
IRB Review Process

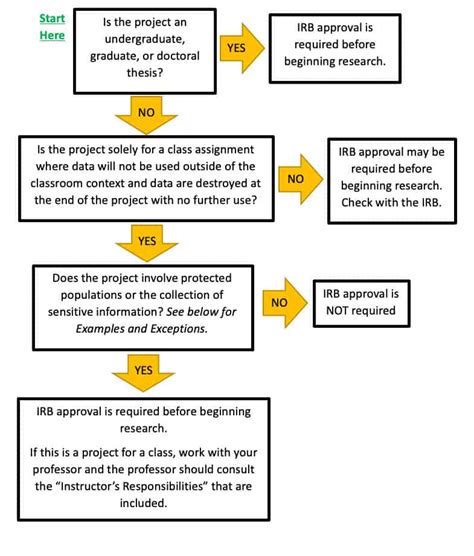
The IRB review process is an essential component of the Emory University IRB guidelines. The process involves the review of research proposals by the IRB to ensure that they meet the requirements for human subjects research. The IRB review process typically involves several steps, including the submission of the research proposal, the review of the proposal by the IRB, and the notification of the researcher regarding the IRB’s decision. The IRB may approve the research proposal, require modifications, or disapprove the proposal. By following the IRB review process, researchers can ensure that their studies are conducted in a manner that is consistent with the highest ethical standards.
Types of IRB Review
There are several types of IRB review, including exempt review, expedited review, and full board review. Exempt review is used for studies that are determined to be exempt from IRB review, such as studies involving existing data or studies that do not involve human subjects. Expedited review is used for studies that involve minimal risk to human subjects, such as studies involving surveys or interviews. Full board review is used for studies that involve more than minimal risk to human subjects, such as studies involving medical interventions or studies that involve vulnerable populations. By understanding the types of IRB review, researchers can determine which type of review is required for their study.
Key Points
- The Emory University IRB guidelines are based on federal regulations, state laws, and institutional policies
- The guidelines cover a wide range of topics, including the principles of informed consent and the protection of vulnerable populations
- The IRB review process involves the review of research proposals by the IRB to ensure that they meet the requirements for human subjects research
- There are several types of IRB review, including exempt review, expedited review, and full board review
- Researchers must follow the Emory University IRB guidelines to ensure that their studies are conducted in a manner that is consistent with the highest ethical standards
Importance of IRB Guidelines
The Emory University IRB guidelines are essential for ensuring that research studies involving human subjects are conducted in a manner that is consistent with the highest ethical standards. The guidelines provide a framework for researchers to follow, and help to ensure that human subjects are protected from harm. By following the IRB guidelines, researchers can ensure that their studies are conducted in a manner that respects the rights and welfare of human subjects, and that the benefits and risks of the study are distributed fairly. Additionally, the IRB guidelines help to ensure that research studies are scientifically valid, and that the results are reliable and generalizable.
Consequences of Non-Compliance
Non-compliance with the Emory University IRB guidelines can have serious consequences, including damage to the reputation of the researcher and the institution, loss of funding, and harm to human subjects. Additionally, non-compliance can result in regulatory action, including fines and penalties. By following the IRB guidelines, researchers can avoid these consequences and ensure that their studies are conducted in a manner that is consistent with the highest ethical standards.
What is the purpose of the Emory University IRB guidelines?
+The purpose of the Emory University IRB guidelines is to ensure that research studies involving human subjects are conducted in a manner that is consistent with the highest ethical standards, and that human subjects are protected from harm.
What types of research are subject to IRB review?
+All research studies involving human subjects are subject to IRB review, including studies involving surveys, interviews, medical interventions, and studies involving vulnerable populations.
What are the consequences of non-compliance with the Emory University IRB guidelines?
+Non-compliance with the Emory University IRB guidelines can result in damage to the reputation of the researcher and the institution, loss of funding, harm to human subjects, and regulatory action, including fines and penalties.
In conclusion, the Emory University IRB guidelines are an essential component of the research process, and are designed to ensure that research studies involving human subjects are conducted in a manner that is consistent with the highest ethical standards. By following the IRB guidelines, researchers can ensure that their studies are conducted in a manner that respects the rights and welfare of human subjects, and that the benefits and risks of the study are distributed fairly. It is essential for researchers to understand the IRB guidelines and to follow them carefully to avoid non-compliance and ensure that their studies are conducted in a manner that is consistent with the highest ethical standards.