Enthalpy change, denoted as ΔH, is a fundamental concept in thermodynamics that represents the total energy change of a system during a chemical reaction or physical transformation. It encompasses both the internal energy change (ΔU) and the energy associated with the work done by the system to its surroundings, typically through the expansion or contraction of gases. Understanding enthalpy change is crucial for predicting the spontaneity, feasibility, and energetic aspects of chemical reactions and processes. In this article, we will explore five ways enthalpy change manifests and is applied in various contexts, highlighting its significance in chemistry, physics, and engineering.
Understanding Enthalpy Change

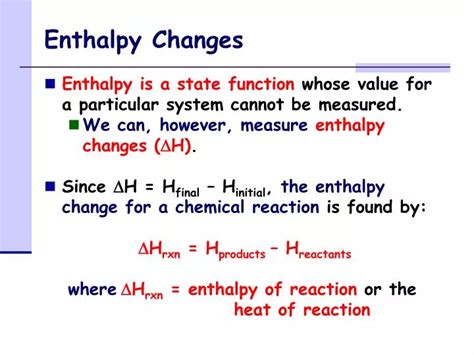
Enthalpy change is calculated using the formula ΔH = ΔU + Δ(PV), where ΔU is the change in internal energy, P is the pressure, and V is the volume. For reactions at constant pressure, which is common in many chemical processes, ΔH = ΔU + PΔV. This equation signifies that the enthalpy change includes not only the change in the internal energy of the system (due to the breaking and forming of bonds) but also the work done by the system against the external pressure. A negative ΔH (exothermic reaction) indicates that the system releases energy to the surroundings, whereas a positive ΔH (endothermic reaction) means the system absorbs energy from the surroundings.
1. Chemical Reactions and Enthalpy Change
In chemical reactions, enthalpy change is a critical parameter for understanding the reaction’s thermodynamics. Exothermic reactions, characterized by a negative ΔH, release heat and are generally spontaneous, meaning they can proceed on their own under the right conditions. Endothermic reactions, with a positive ΔH, absorb heat and are non-spontaneous, requiring an external energy source to proceed. The magnitude of ΔH provides insight into the reaction’s energy requirements or releases, guiding the design of processes to either harness the released energy or provide the necessary energy for the reaction to occur.
| Type of Reaction | Enthalpy Change (ΔH) | Spontaneity |
|---|---|---|
| Exothermic | Negative | Spontaneous |
| Endothermic | Positive | Non-spontaneous |
2. Phase Transitions and Enthalpy Change
Enthalpy change is also significant during phase transitions, such as melting, boiling, and sublimation. Each phase transition has an associated enthalpy change, known as the enthalpy of fusion (for melting), enthalpy of vaporization (for boiling), and enthalpy of sublimation. These values are positive, indicating that energy must be supplied to the system for the phase transition to occur. For instance, the enthalpy of vaporization of water is approximately 40.65 kJ/mol at 100°C and 1 atm, meaning that 40.65 kJ of energy is required to vaporize one mole of water at these conditions.
3. Enthalpy Change in Biological Systems
In biological systems, enthalpy change plays a vital role in metabolic pathways and biochemical reactions. Enzymes, biological catalysts, lower the activation energy required for reactions to proceed, thereby influencing the enthalpy change. The efficiency and direction of metabolic pathways are determined by the enthalpy changes of the individual reactions within the pathway. For example, the breakdown of ATP (adenosine triphosphate) to ADP (adenosine diphosphate) and inorganic phosphate has a negative ΔH, releasing energy that can be used by the cell for various processes.
4. Enthalpy Change in Industrial Processes
Industrial processes, such as chemical synthesis, petroleum refining, and power generation, rely heavily on the management of enthalpy changes. In chemical synthesis, controlling the enthalpy change of reactions is crucial for optimizing yield, selectivity, and energy efficiency. In power generation, particularly in steam turbines, the enthalpy change of water from liquid to vapor and back to liquid is harnessed to produce mechanical work, which is then converted into electrical energy.
5. Enthalpy Change and Environmental Considerations
Lastly, understanding enthalpy change is important for assessing the environmental impact of industrial and natural processes. The enthalpy changes associated with the combustion of fossil fuels, for instance, contribute to global warming through the release of greenhouse gases. Similarly, the enthalpy of fusion and vaporization of polar ice caps influences the Earth’s energy balance and climate. Thus, considering enthalpy changes in the context of energy production, consumption, and environmental conservation is essential for developing sustainable practices and mitigating climate change.
Key Points
- Enthalpy change (ΔH) is a measure of the total energy change in a system during a chemical reaction or physical transformation.
- It is crucial for understanding the spontaneity and feasibility of chemical reactions and processes.
- Enthalpy change is significant in chemical reactions, phase transitions, biological systems, industrial processes, and environmental considerations.
- Managing enthalpy change is essential for optimizing energy efficiency, yield, and selectivity in various processes.
- Understanding enthalpy change is vital for addressing environmental challenges and developing sustainable practices.
In conclusion, enthalpy change is a fundamental concept that underlies various aspects of science and engineering, from the smallest biochemical reactions to large-scale industrial processes and environmental phenomena. Its significance extends beyond mere theoretical interest, offering practical insights into the design, optimization, and sustainability of processes that shape our world.
What is the difference between internal energy change (ΔU) and enthalpy change (ΔH)?
+Internal energy change (ΔU) refers to the change in the total energy of a system, excluding the energy associated with the work done by the system to its surroundings. Enthalpy change (ΔH), on the other hand, includes both the internal energy change and the energy associated with the work done by the system, typically through the expansion or contraction of gases.
How does enthalpy change influence the spontaneity of chemical reactions?
+A negative enthalpy change (ΔH) indicates an exothermic reaction, which is generally spontaneous. A positive ΔH signifies an endothermic reaction, which is non-spontaneous and requires an external energy source to proceed.
What role does enthalpy change play in industrial processes?
+Enthalpy change is crucial in industrial processes for optimizing energy efficiency, yield, and selectivity. It guides the design of reactions, separation processes, and energy management systems to achieve desired outcomes efficiently.



