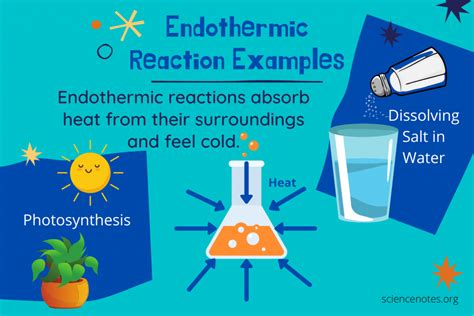
Endothermic reactions are a fundamental concept in chemistry, referring to processes where energy is absorbed from the surroundings to drive a chemical reaction. These reactions are characterized by an increase in temperature, and they play a crucial role in various industrial, biological, and environmental processes. In this article, we will delve into the world of endothermic reactions, exploring their definition, examples, and significance in different fields.
Key Points
- Endothermic reactions absorb energy from the surroundings to drive a chemical reaction
- Examples of endothermic reactions include photosynthesis, the decomposition of ammonia, and the production of cement
- Endothermic reactions have various applications in industries such as agriculture, construction, and pharmaceuticals
- Understanding endothermic reactions is essential for developing efficient and sustainable processes
- Endothermic reactions can be influenced by factors such as temperature, pressure, and catalysts
Definition and Characteristics of Endothermic Reactions
Endothermic reactions are defined as chemical reactions that absorb energy from the surroundings in the form of heat, light, or other forms of energy. This energy is used to break chemical bonds, form new bonds, or initiate other chemical processes. The energy absorbed during an endothermic reaction is typically measured in terms of the change in enthalpy (ΔH), which is a thermodynamic property that represents the total energy of a system. A positive ΔH value indicates an endothermic reaction, while a negative ΔH value indicates an exothermic reaction.
Examples of Endothermic Reactions
There are numerous examples of endothermic reactions that occur in various fields, including:
- Photosynthesis: This process involves the conversion of carbon dioxide and water into glucose and oxygen, using energy from sunlight. Photosynthesis is a complex endothermic reaction that occurs in plants, algae, and some bacteria.
- Decomposition of ammonia: The decomposition of ammonia (NH3) into nitrogen (N2) and hydrogen (H2) is an endothermic reaction that requires energy to break the chemical bonds. This reaction is used in the production of fertilizers and other chemicals.
- Production of cement: The production of cement involves the calcination of limestone (CaCO3) to produce calcium oxide (CaO) and carbon dioxide (CO2). This endothermic reaction requires energy to break the chemical bonds and is used in the construction industry.
- Evaporation of water: The evaporation of water is an endothermic reaction that occurs when water is heated, causing the molecules to gain energy and change from a liquid to a gas state.
- Nitrogen fixation: Nitrogen fixation is an endothermic reaction that involves the conversion of nitrogen (N2) into ammonia (NH3) or other nitrogen-containing compounds. This reaction is used in the production of fertilizers and is essential for plant growth.
| Reaction | ΔH (kJ/mol) |
|---|---|
| Photosynthesis | 470 |
| Decomposition of ammonia | 45.9 |
| Production of cement | 167 |
| Evaporation of water | 40.7 |
| Nitrogen fixation | 27.7 |
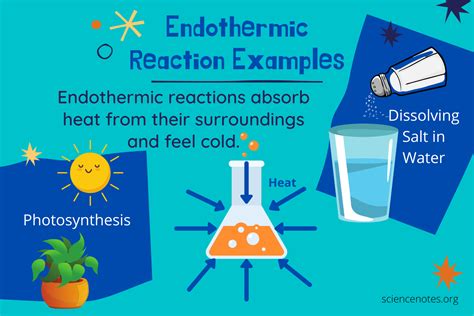
Applications of Endothermic Reactions
Endothermic reactions have numerous applications in various fields, including:
- Agriculture: Endothermic reactions are used in the production of fertilizers, such as ammonia and nitric acid, which are essential for plant growth.
- Construction: The production of cement, which involves an endothermic reaction, is used in the construction industry for building and infrastructure development.
- Pharmaceuticals: Endothermic reactions are used in the synthesis of various pharmaceutical compounds, such as antibiotics and vaccines.
- Energy storage: Endothermic reactions can be used for energy storage, such as in the production of hydrogen fuel cells.
- Environmental remediation: Endothermic reactions can be used to clean up environmental pollutants, such as the degradation of organic pollutants in soil and water.
Influence of Factors on Endothermic Reactions
Endothermic reactions can be influenced by various factors, including:
- Temperature: Increasing the temperature can increase the rate of an endothermic reaction, but it can also lead to the formation of unwanted byproducts.
- Pressure: Increasing the pressure can increase the rate of an endothermic reaction, but it can also lead to the formation of unwanted byproducts.
- Catalysts: Catalysts can increase the rate of an endothermic reaction by providing an alternative reaction pathway with a lower activation energy.
- Concentration: The concentration of reactants can influence the rate of an endothermic reaction, with higher concentrations leading to faster reaction rates.
What is the difference between endothermic and exothermic reactions?
+Endothermic reactions absorb energy from the surroundings, while exothermic reactions release energy to the surroundings.
What are some examples of endothermic reactions in everyday life?
+Examples of endothermic reactions in everyday life include the evaporation of water, the melting of ice, and the cooking of food.
How can endothermic reactions be optimized and controlled?
+Endothermic reactions can be optimized and controlled by adjusting factors such as temperature, pressure, and catalysts, as well as by using various techniques such as kinetics and thermodynamics.
In conclusion, endothermic reactions are an essential part of various chemical processes, and understanding their definition, characteristics, and applications is crucial for optimizing and controlling these reactions in different fields. By recognizing the significance of endothermic reactions and their influence on various industries, we can develop more efficient and sustainable processes that benefit society and the environment.