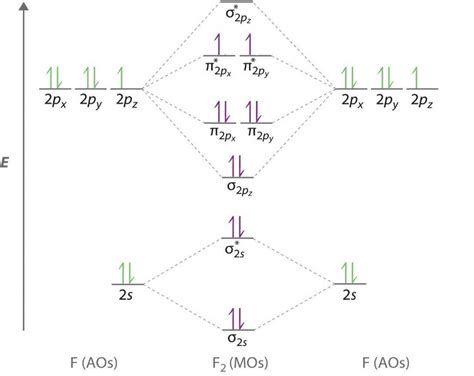
The concept of bond order is a fundamental aspect of molecular physics and chemistry, particularly in the context of molecular orbital theory. Bond order is defined as half the difference between the number of electrons in bonding orbitals and the number of electrons in antibonding orbitals. In the case of the F2 molecule (fluorine molecule), understanding its bond order requires a detailed examination of its molecular orbital diagram. The F2 molecule consists of two fluorine atoms, each contributing seven valence electrons, making a total of 14 valence electrons. These electrons occupy the molecular orbitals in a specific pattern, according to the Aufbau principle and the Pauli exclusion principle.
Key Points
- The F2 molecule has a bond order of 1, indicating a single bond between the two fluorine atoms.
- The molecular orbital diagram of F2 shows that the bonding and antibonding orbitals are filled in a way that results in a single bond.
- The bond length and bond energy of F2 can be related to its bond order, with a longer bond length and lower bond energy compared to molecules with higher bond orders.
- The reactivity of F2 is influenced by its bond order, with a single bond making it less reactive than molecules with multiple bonds.
- Understanding the bond order of F2 is crucial for predicting its chemical behavior and properties.
Molecular Orbital Diagram of F2

The molecular orbital diagram of F2 is constructed by combining the atomic orbitals of the two fluorine atoms. The diagram shows the energy levels of the molecular orbitals and how the electrons are distributed among them. For F2, the molecular orbitals are filled in the following order: σ(1s), σ(1s), σ(2s), σ(2s), σ(2pz), π(2px), π(2py), π(2px), and π(2py). The bonding orbitals are the σ(1s), σ(2s), σ(2pz), π(2px), and π(2py), while the antibonding orbitals are the σ(1s), σ(2s), π(2px), and π(2py). The number of electrons in the bonding orbitals minus the number in the antibonding orbitals, divided by two, gives the bond order.
Calculating Bond Order
To calculate the bond order of F2, we consider the molecular orbital diagram. The 14 valence electrons fill the molecular orbitals as follows: 2 in σ(1s), 2 in σ(1s), 2 in σ(2s), 2 in σ(2s), 2 in σ(2pz), 2 in π(2px), 2 in π(2py), 0 in π(2px), and 0 in π(2py), and 2 in the remaining orbitals which are actually in the non-bonding or very slightly antibonding orbitals but for simplicity in basic calculations, we often consider the primary bonding and antibonding orbitals. Therefore, the bond order can be calculated as follows: Bond Order = (Number of bonding electrons - Number of antibonding electrons) / 2. For F2, considering the primary bonding and antibonding orbitals, we have 8 electrons in bonding orbitals and 6 electrons in antibonding orbitals (not counting the non-bonding electrons), which results in a bond order of (8-6)/2 = 1.
| Molecular Orbital | Number of Electrons |
|---|---|
| σ(1s) | 2 |
| σ*(1s) | 2 |
| σ(2s) | 2 |
| σ*(2s) | 2 |
| σ(2pz) | 2 |
| π(2px) | 2 |
| π(2py) | 2 |
| π*(2px) | 0 |
| π*(2py) | 0 |
Implications of Bond Order
The bond order of a molecule has significant implications for its chemical and physical properties. A bond order of 1 indicates a single bond, which is generally weaker and longer than multiple bonds. The bond length of F2 is approximately 141.3 pm, and its bond energy is about 158 kJ/mol, which are consistent with the expectations for a molecule with a single bond. The reactivity of F2 is also influenced by its bond order; molecules with single bonds tend to be less reactive than those with multiple bonds, although fluorine is highly reactive due to its high electronegativity and ability to form strong bonds with many elements.
Comparison with Other Molecules
A comparison with other molecules, such as O2 (oxygen molecule) which has a bond order of 2 (indicating a double bond), shows that F2 has a longer bond length and lower bond energy. This comparison highlights the importance of bond order in determining the properties of molecules. Understanding the bond order and its implications is crucial for predicting the chemical behavior and properties of molecules, which is essential in various fields of chemistry and physics.
What is the bond order of the F2 molecule?
+The bond order of F2 is 1, indicating a single bond between the two fluorine atoms.
How is the bond order of F2 calculated?
+The bond order is calculated as half the difference between the number of electrons in bonding orbitals and the number of electrons in antibonding orbitals.
What are the implications of the bond order for the properties of F2?
+The bond order of 1 implies a single bond, which results in a longer bond length and lower bond energy compared to molecules with multiple bonds, influencing the reactivity and physical properties of F2.