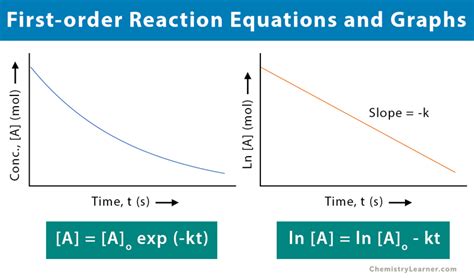
The realm of chemical kinetics is replete with complex phenomena, but few are as fundamental as the first order reaction. This type of reaction is characterized by its dependence on the concentration of a single reactant, making it a cornerstone of understanding in the field of chemistry. The first order reaction is a paradigm of simplicity and elegance, where the rate of reaction is directly proportional to the concentration of the reactant. This proportionality is expressed through the rate equation, which is a mathematical representation of the relationship between the rate of reaction and the concentration of the reactant.
In a first order reaction, the rate of disappearance of the reactant is given by the equation: rate = k[A], where k is the rate constant and [A] is the concentration of the reactant. This equation is a testament to the simplicity and predictability of first order reactions, as the rate of reaction can be easily calculated if the concentration of the reactant and the rate constant are known. The rate constant, k, is a measure of the likelihood of a reactant molecule undergoing a chemical transformation, and its value is independent of the concentration of the reactant.
Key Points
- The rate of a first order reaction is directly proportional to the concentration of the reactant.
- The rate equation for a first order reaction is rate = k[A], where k is the rate constant and [A] is the concentration of the reactant.
- The half-life of a first order reaction is independent of the initial concentration of the reactant.
- First order reactions are characterized by a linear decrease in the concentration of the reactant over time.
- The integrated rate law for a first order reaction is ln([A]t/[A]0) = -kt, where [A]t is the concentration of the reactant at time t and [A]0 is the initial concentration of the reactant.
Characteristics of First Order Reactions
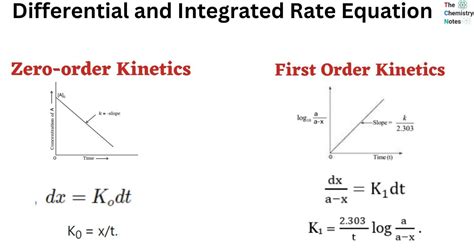
One of the most distinctive features of first order reactions is their half-life, which is the time it takes for the concentration of the reactant to decrease by half. The half-life of a first order reaction is independent of the initial concentration of the reactant, making it a useful parameter for characterizing the reaction. This independence is a direct result of the exponential nature of the concentration decrease, which is a hallmark of first order reactions.
Integrated Rate Law
The integrated rate law for a first order reaction is a mathematical expression that describes the relationship between the concentration of the reactant and time. The integrated rate law is given by the equation: ln([A]t/[A]0) = -kt, where [A]t is the concentration of the reactant at time t and [A]0 is the initial concentration of the reactant. This equation is a powerful tool for analyzing first order reactions, as it allows for the calculation of the concentration of the reactant at any given time.
| Reaction Type | Rate Equation | Integrated Rate Law |
|---|---|---|
| First Order | rate = k[A] | ln([A]t/[A]0) = -kt |
| Second Order | rate = k[A]^2 | 1/[A]t - 1/[A]0 = kt |
| Zero Order | rate = k | [A]t - [A]0 = -kt |

Applications of First Order Reactions
First order reactions have a wide range of applications in various fields, including chemistry, biology, and pharmacology. One of the most significant applications of first order reactions is in the field of chemical synthesis, where they are used to produce a wide range of compounds, from simple molecules to complex pharmaceuticals. First order reactions are also used in the development of new drugs, where they are used to study the pharmacokinetics and pharmacodynamics of new compounds.
Pharmaceutical Development
In pharmaceutical development, first order reactions are used to study the absorption, distribution, metabolism, and excretion (ADME) of new compounds. The half-life of a drug is a critical parameter in determining its efficacy and safety, and first order reactions provide a powerful tool for calculating this parameter. By understanding the kinetics of a drug, pharmaceutical companies can optimize its formulation and dosing regimen, leading to improved efficacy and reduced side effects.
In conclusion, first order reactions are a fundamental concept in chemical kinetics, with a wide range of applications in various fields. Their simplicity and predictability make them a powerful tool for understanding complex chemical phenomena, and their applications in pharmaceutical development and chemical synthesis are particularly significant. By understanding the characteristics and applications of first order reactions, scientists and engineers can develop new compounds and processes that improve our daily lives.
What is the definition of a first order reaction?
+A first order reaction is a chemical reaction in which the rate of reaction is directly proportional to the concentration of a single reactant.
What is the half-life of a first order reaction?
+The half-life of a first order reaction is the time it takes for the concentration of the reactant to decrease by half, and it is independent of the initial concentration of the reactant.
What are the applications of first order reactions in pharmaceutical development?
+First order reactions are used in pharmaceutical development to study the pharmacokinetics and pharmacodynamics of new compounds, and to optimize their formulation and dosing regimen.