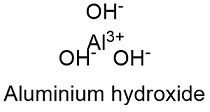
The aluminium hydroxide formula, also known as aluminum hydroxide, is a compound that is widely used in various industrial, pharmaceutical, and cosmetic applications. The chemical formula for aluminium hydroxide is Al(OH)3 or Al2O3·3H2O, indicating that it consists of aluminium, oxygen, and hydrogen atoms. This compound is often found in nature as the mineral gibbsite, one of the main components of bauxite, the primary ore used to produce aluminium metal.
Chemical Structure and Properties

Aluminium hydroxide has a molecular weight of 78.00 g/mol and a density of approximately 2.42 g/cm3. It is a white, amorphous powder that is insoluble in water but soluble in acids and alkalies. The compound has a number of distinct properties, including its ability to act as an antacid, astringent, and antiperspirant, which makes it a common ingredient in various products such as antacids, skin care products, and deodorants.
Industrial and Pharmaceutical Applications
In the pharmaceutical industry, aluminium hydroxide is used as an active ingredient in antacids, where it helps to neutralize stomach acid and relieve symptoms of heartburn and indigestion. It is also used as an adjuvant in vaccines, where it helps to stimulate the immune system and enhance the body’s response to the vaccine. In addition, aluminium hydroxide is used in the treatment of hyperphosphatemia, a condition characterized by elevated levels of phosphate in the blood.
| Application | Description |
|---|---|
| Pharmaceuticals | Antacids, vaccines, treatment of hyperphosphatemia |
| Cosmetics | Skin care products, deodorants, antiperspirants |
| Industrial | Water treatment, paper coating, ceramics |
Preparation and Synthesis
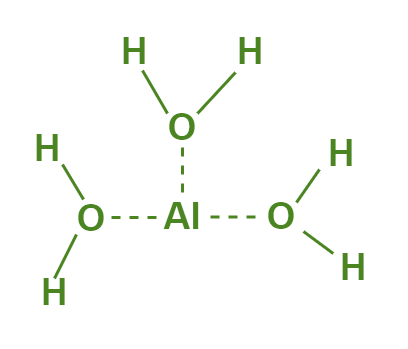
Aluminium hydroxide can be prepared through the reaction of aluminium sulfate with sodium hydroxide, followed by the precipitation of the resulting aluminium hydroxide. The compound can also be synthesized through the hydrolysis of aluminium salts, such as aluminium chloride or aluminium nitrate. The purity and properties of the resulting aluminium hydroxide can vary depending on the method of preparation and the conditions used.
Safety and Handling
Aluminium hydroxide is generally considered to be safe and non-toxic, but it can cause skin and eye irritation in some individuals. It is also important to handle the compound with care, as it can be a respiratory irritant if inhaled. In addition, aluminium hydroxide can react with certain acids and bases to produce hazardous compounds, so it is essential to follow proper safety protocols when handling the compound.
Key Points
- The aluminium hydroxide formula is Al(OH)3 or Al2O3·3H2O, indicating that it consists of aluminium, oxygen, and hydrogen atoms.
- The compound has a number of distinct properties, including its ability to act as an antacid, astringent, and antiperspirant.
- Aluminium hydroxide is used in various industrial, pharmaceutical, and cosmetic applications, including antacids, vaccines, skin care products, and deodorants.
- The compound can be prepared through the reaction of aluminium sulfate with sodium hydroxide or through the hydrolysis of aluminium salts.
- Aluminium hydroxide is generally considered to be safe and non-toxic, but it can cause skin and eye irritation in some individuals and should be handled with care.
Environmental and Health Considerations
Aluminium hydroxide has been shown to have a number of environmental and health benefits, including its ability to remove impurities from water and its use as a vaccine adjuvant. However, there are also concerns about the potential health effects of long-term exposure to aluminium, particularly in relation to neurological disorders such as Alzheimer’s disease. Further research is needed to fully understand the potential risks and benefits of aluminium hydroxide and to ensure its safe and effective use in various applications.
What is the aluminium hydroxide formula?
+The aluminium hydroxide formula is Al(OH)3 or Al2O3·3H2O, indicating that it consists of aluminium, oxygen, and hydrogen atoms.
What are the main applications of aluminium hydroxide?
+Aluminium hydroxide is used in various industrial, pharmaceutical, and cosmetic applications, including antacids, vaccines, skin care products, and deodorants.
Is aluminium hydroxide safe and non-toxic?
+Aluminium hydroxide is generally considered to be safe and non-toxic, but it can cause skin and eye irritation in some individuals and should be handled with care.