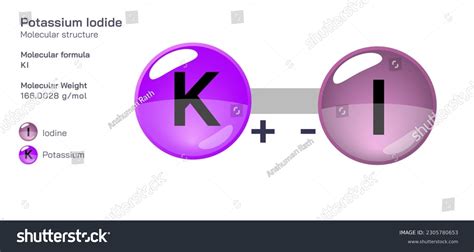
The potassium iodide formula is a chemical compound that has been widely used in various applications, including as a source of iodine, a catalyst in chemical reactions, and a contrast agent in medical imaging. To understand the properties and applications of potassium iodide, it is essential to examine its chemical structure and formula. The potassium iodide formula is KI, which consists of one potassium (K) atom and one iodine (I) atom. This formula indicates that potassium iodide is a binary compound, composed of two elements.
Chemical Structure and Properties

The chemical structure of potassium iodide is characterized by an ionic bond between the potassium and iodine atoms. The potassium atom loses one electron to form a positively charged ion (K+), while the iodine atom gains one electron to form a negatively charged ion (I-). The resulting compound is a white, crystalline solid that is highly soluble in water. Potassium iodide has a molecular weight of 166.00 g/mol and a melting point of 681°C. Its chemical properties make it a useful reagent in various chemical reactions, including the production of iodine and the synthesis of pharmaceuticals.
Key Points
- The potassium iodide formula is KI, consisting of one potassium atom and one iodine atom.
- Potassium iodide is a binary compound with an ionic bond between the potassium and iodine atoms.
- The compound has a molecular weight of 166.00 g/mol and a melting point of 681°C.
- Potassium iodide is highly soluble in water and is used as a source of iodine, a catalyst in chemical reactions, and a contrast agent in medical imaging.
- The chemical properties of potassium iodide make it a useful reagent in various chemical reactions, including the production of iodine and the synthesis of pharmaceuticals.
Applications of Potassium Iodide
Potassium iodide has a range of applications due to its unique chemical properties. One of its primary uses is as a source of iodine, which is essential for the production of thyroid hormones. Potassium iodide is also used as a catalyst in chemical reactions, such as the synthesis of pharmaceuticals and the production of dyes. In medical imaging, potassium iodide is used as a contrast agent to enhance the visibility of structures and lesions. Additionally, potassium iodide is used in nuclear medicine to treat thyroid cancer and to protect the thyroid gland from radiation damage.
| Application | Description |
|---|---|
| Source of Iodine | Potassium iodide is used as a source of iodine, which is essential for the production of thyroid hormones. |
| Catalyst in Chemical Reactions | Potassium iodide is used as a catalyst in chemical reactions, such as the synthesis of pharmaceuticals and the production of dyes. |
| Contrast Agent in Medical Imaging | Potassium iodide is used as a contrast agent to enhance the visibility of structures and lesions in medical imaging. |
| Nuclear Medicine | Potassium iodide is used in nuclear medicine to treat thyroid cancer and to protect the thyroid gland from radiation damage. |

Preparation and Handling of Potassium Iodide
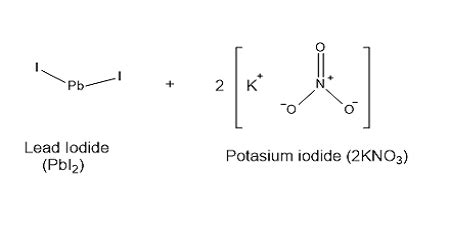
Potassium iodide can be prepared by reacting potassium hydroxide with iodine in water. The resulting solution is then crystallized to produce pure potassium iodide. When handling potassium iodide, it is essential to take precautions to avoid exposure to the skin and eyes, as it can cause irritation and burns. Additionally, potassium iodide should be stored in a cool, dry place to prevent decomposition and contamination.
Safety Considerations
While potassium iodide is generally considered safe when handled properly, there are potential risks associated with its use. Prolonged exposure to potassium iodide can cause skin and eye irritation, and inhalation of the dust can lead to respiratory problems. In rare cases, potassium iodide can cause allergic reactions, including anaphylaxis. It is essential to follow proper handling and safety protocols when working with potassium iodide to minimize the risk of adverse effects.
What is the molecular weight of potassium iodide?
+The molecular weight of potassium iodide is 166.00 g/mol.
What are the common applications of potassium iodide?
+Potassium iodide is used as a source of iodine, a catalyst in chemical reactions, and a contrast agent in medical imaging. It is also used in nuclear medicine to treat thyroid cancer and to protect the thyroid gland from radiation damage.
How should potassium iodide be handled and stored?
+Potassium iodide should be handled with care to avoid exposure to the skin and eyes. It should be stored in a cool, dry place to prevent decomposition and contamination.
Meta Description: Learn about the potassium iodide formula, its chemical structure, properties, and applications. Discover the uses of potassium iodide as a source of iodine, catalyst, and contrast agent, and understand the safety considerations for handling and storing this compound.