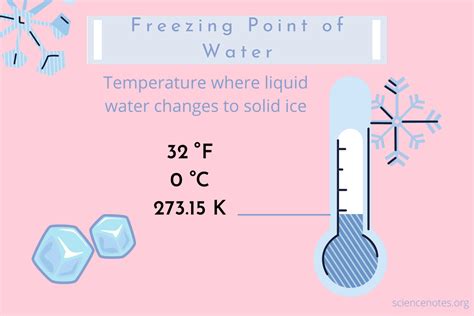
The freezing point of water is a fundamental physical constant that has numerous implications in various fields, including science, engineering, and everyday life. At standard atmospheric pressure, water freezes at a temperature of 0 degrees Celsius (°C) or 32 degrees Fahrenheit (°F). This temperature mark is not only a reference point for the Celsius scale but also a critical value in understanding the behavior of water under different conditions. Here are five key facts about the freezing point of water at 0°C:
Introduction to Freezing Point
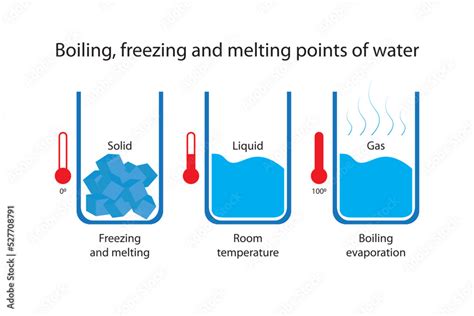
The freezing point is the temperature at which a liquid changes state to become a solid. For water, this process occurs when the molecules slow down enough to come together in a crystalline structure, forming ice. The exact temperature at which water freezes can vary slightly depending on the purity of the water and the pressure it is under. However, under standard conditions, 0°C is the universally accepted freezing point of water.
Freezing Point and Pressure
It’s worth noting that the freezing point of water can be affected by pressure. At higher pressures, the freezing point of water can be slightly lower than 0°C. This phenomenon is crucial in certain natural and industrial processes. For instance, the pressure at the bottom of a deep body of water can be high enough to lower the freezing point, potentially preventing the water from freezing even if the temperature is below 0°C. This effect, however, is more significant in the context of seawater, where the presence of salts and other substances also influences the freezing point.
Key Points
- The freezing point of water is 0°C at standard atmospheric pressure.
- The exact freezing point can be influenced by factors such as purity and pressure.
- Water expands as it freezes, which is why ice floats on liquid water.
- The freezing point of water is a critical reference point for the Celsius temperature scale.
- Understanding the freezing point of water is essential in various fields, including chemistry, physics, and engineering.
Physical Properties of Water at Freezing Point

When water freezes, it undergoes a phase transition from liquid to solid. During this process, the physical properties of water change significantly. One of the most notable changes is the increase in volume; ice occupies about 9% more volume than the same mass of liquid water. This is why ice floats on top of liquid water, a phenomenon critical for many ecological and environmental processes. The density change also has implications for aquatic life and the structure of lakes and rivers during winter months.
Importance of Freezing Point in Daily Life
The freezing point of water has numerous practical implications in daily life. From the preservation of food to the operation of heating and cooling systems, understanding the behavior of water at its freezing point is crucial. In cold climates, the freezing point of water dictates the design and maintenance of infrastructure, such as roads, bridges, and buildings, to withstand the expansion of water as it freezes. Additionally, the freezing point of water is a key factor in meteorology, influencing weather patterns and the formation of precipitation.
| Property | Value |
|---|---|
| Freezing Point of Water | 0°C |
| Density of Ice | Approximately 0.92 g/cm³ |
| Density of Liquid Water | Approximately 1.00 g/cm³ |

Conclusion and Future Implications
In conclusion, the freezing point of water at 0°C is a fundamental constant with far-reaching implications. From the basic principles of physical science to the complexities of ecological systems, the behavior of water at its freezing point plays a critical role. As science and technology continue to evolve, understanding the freezing point of water and its related phenomena will remain essential for advancing our knowledge and addressing the challenges posed by an ever-changing environment.
What is the freezing point of water in Celsius?
+The freezing point of water is 0°C at standard atmospheric pressure.
How does pressure affect the freezing point of water?
+Higher pressures can lower the freezing point of water slightly, which is significant in certain natural and industrial processes.
Why does ice float on liquid water?
+Ice floats because it is less dense than liquid water, expanding by about 9% in volume when water freezes.