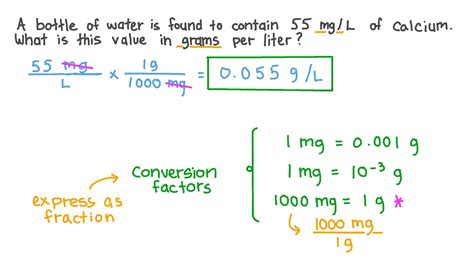
Converting grams to liters is a fundamental process in various fields, including chemistry, physics, and engineering. This conversion is essential when dealing with substances that have different densities, as the weight of a substance (in grams) does not directly translate to its volume (in liters). Understanding the relationship between grams and liters requires a basic comprehension of density, which is defined as mass per unit volume. The formula for density is density = mass/volume, or ρ = m/V, where ρ is the density, m is the mass, and V is the volume.
To convert grams to liters, one must know the density of the substance in question. The density of a substance is typically expressed in units of grams per milliliter (g/mL) or grams per cubic centimeter (g/cm³), which are equivalent. Since 1 liter (L) equals 1,000 milliliters (mL) or 1,000 cubic centimeters (cm³), the conversion can be straightforward once the density is known. For example, if you have a substance with a density of 0.5 g/mL and you want to convert 500 grams of this substance into liters, you would first determine the volume in milliliters and then convert that volume to liters.
Key Points
- The conversion of grams to liters depends on the density of the substance.
- Density is calculated as mass per unit volume (ρ = m/V).
- Knowing the density allows for the calculation of volume from mass.
- The formula to find volume from mass and density is V = m/ρ.
- 1 liter equals 1,000 milliliters or 1,000 cubic centimeters.
Understanding Density and Its Role in Conversion
Density plays a critical role in the conversion process because it provides the link between mass (in grams) and volume (in liters). Different substances have different densities due to their unique molecular structures and how their molecules pack together. For instance, water has a density of approximately 1 g/mL at standard temperature and pressure (STP) conditions, meaning that 1 gram of water occupies a volume of 1 milliliter. In contrast, substances like oils or alcohols have different densities and thus will occupy different volumes when compared to the same mass of water.
Calculating Volume from Mass and Density
The formula to calculate the volume of a substance when its mass and density are known is V = m/ρ, where V is the volume, m is the mass, and ρ is the density. This formula rearranges the density equation (ρ = m/V) to solve for volume. For example, if you have 200 grams of a substance with a density of 0.8 g/mL and you want to find its volume in liters, you would first calculate the volume in milliliters: V = 200 g / 0.8 g/mL = 250 mL. Then, to convert milliliters to liters, you divide by 1,000, as there are 1,000 milliliters in a liter: 250 mL / 1,000 = 0.25 L.
| Substance | Density (g/mL) | Mass (g) | Volume (mL) | Volume (L) |
|---|---|---|---|---|
| Water | 1 | 500 | 500 | 0.5 |
| Oil | 0.9 | 500 | 555.56 | 0.5556 |
| Alcohol | 0.8 | 200 | 250 | 0.25 |

Practical Applications and Considerations

The ability to convert grams to liters is not only theoretically important but also practically useful in various scenarios. In cooking, for instance, recipes often list ingredients in grams, but to mix or measure these ingredients accurately, one needs to know their volumes. Similarly, in chemistry labs, precise measurements of substances in both mass and volume are critical for experiments and reactions. Understanding how to convert between these units can help in preparing solutions, calculating concentrations, and ensuring the accuracy of experimental results.
In industrial settings, such as in the production of beverages or pharmaceuticals, the conversion between grams and liters is essential for quality control, packaging, and distribution. Manufacturers must accurately measure the mass and volume of their products to ensure they meet regulatory standards and customer expectations. This requires not only a good understanding of the conversion process but also the ability to work with different units and densities.
Addressing Common Challenges
One common challenge in converting grams to liters is dealing with substances that have densities close to 1 g/mL, such as water or certain aqueous solutions. In these cases, the mass and volume are nearly equivalent, making it easy to approximate volumes. However, for substances with significantly different densities, even small errors in measurement or calculation can lead to substantial discrepancies between the actual and calculated volumes.
Another challenge is the potential for confusion between units, especially when working with both metric and imperial systems. It's essential to ensure that all measurements are in compatible units before performing calculations. Lastly, the effects of temperature and pressure on density should not be overlooked, especially in applications where precision is paramount.
What is the formula to convert grams to liters?
+The formula to convert grams to liters involves knowing the density of the substance. The volume (V) in liters can be calculated using the formula V = m/ρ, where m is the mass in grams and ρ is the density in g/mL. Then, to convert milliliters to liters, divide by 1,000.
Why is density important in the conversion process?
+Density is crucial because it varies among substances, affecting how much volume a given mass of the substance occupies. Without knowing the density, one cannot accurately convert grams to liters.
How do temperature and pressure affect the conversion?
+Temperature and pressure can affect the density of a substance, thereby affecting the conversion from grams to liters. For precise calculations, especially in scientific or industrial applications, it's essential to consider these factors and use the density value relevant to the specific conditions.
In conclusion, converting grams to liters is a process that requires an understanding of density and its role in linking mass and volume. By applying the formula V = m/ρ and considering the specific conditions under which the conversion is being made, individuals can accurately calculate the volume of a substance in liters from its mass in grams. This skill is essential in various fields, from cooking and chemistry to industrial manufacturing, and its application can ensure precision, quality, and safety in numerous contexts.