The Gibbs free energy calculation is a fundamental concept in thermodynamics, used to determine the spontaneity of a chemical reaction. It is a measure of the maximum amount of work that can be extracted from a system at constant temperature and pressure. In this article, we will delve into the world of Gibbs free energy calculations, exploring the underlying principles, equations, and applications of this crucial concept.
Key Points
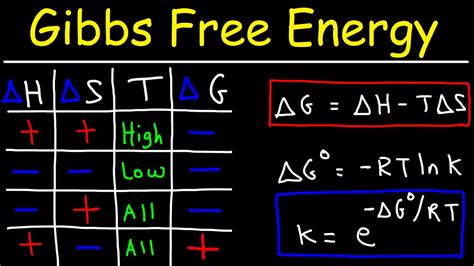
- The Gibbs free energy equation is given by ΔG = ΔH - TΔS, where ΔG is the change in Gibbs free energy, ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy.
- A negative ΔG value indicates a spontaneous reaction, while a positive ΔG value indicates a non-spontaneous reaction.
- The standard Gibbs free energy change (ΔG°) is a measure of the spontaneity of a reaction under standard conditions.
- Gibbs free energy calculations are essential in understanding the thermodynamics of chemical reactions, including phase transitions, solubility, and biochemical processes.
- Accurate calculations of Gibbs free energy require precise values of enthalpy, entropy, and temperature.
Introduction to Gibbs Free Energy
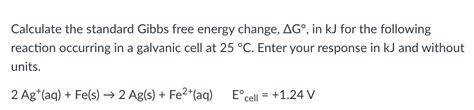
The concept of Gibbs free energy was first introduced by Josiah Willard Gibbs in the late 19th century. It is defined as the maximum amount of work that can be extracted from a system at constant temperature and pressure. The Gibbs free energy equation is given by ΔG = ΔH - TΔS, where ΔG is the change in Gibbs free energy, ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy.
Enthalpy and Entropy Changes
Enthalpy (H) is a measure of the total energy of a system, including the internal energy (U) and the energy associated with the pressure and volume of the system. Entropy (S) is a measure of the disorder or randomness of a system. Changes in enthalpy (ΔH) and entropy (ΔS) are crucial in determining the spontaneity of a chemical reaction. A negative ΔH value indicates an exothermic reaction, while a positive ΔH value indicates an endothermic reaction. A positive ΔS value indicates an increase in disorder, while a negative ΔS value indicates a decrease in disorder.
| Thermodynamic Property | Definition | Unit |
|---|---|---|
| Enthalpy (H) | Measure of total energy | Joules (J) |
| Entropy (S) | Measure of disorder or randomness | Joules per Kelvin (J/K) |
| Gibbs Free Energy (G) | Measure of maximum work extractable | Joules (J) |
Standard Gibbs Free Energy Change

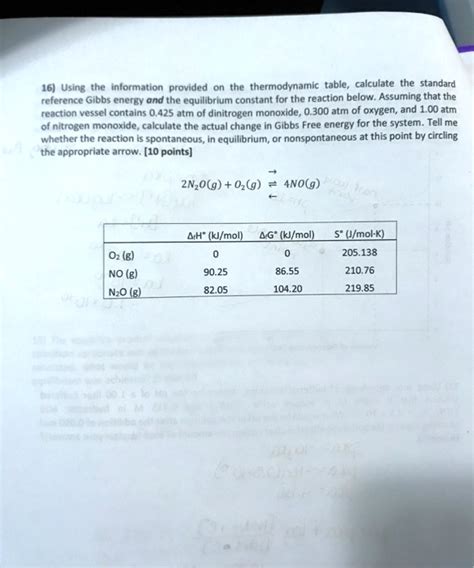
The standard Gibbs free energy change (ΔG°) is a measure of the spontaneity of a reaction under standard conditions. Standard conditions are defined as 1 atm pressure, 25°C temperature, and 1 M concentration for all reactants and products. The standard Gibbs free energy change is related to the equilibrium constant (K) of a reaction by the equation ΔG° = -RT ln K, where R is the gas constant and T is the temperature in Kelvin.
Calculating Gibbs Free Energy
To calculate the Gibbs free energy of a reaction, we need to know the changes in enthalpy and entropy of the reaction. These values can be obtained from experimental data or calculated using theoretical models. The Gibbs free energy equation can be used to calculate the spontaneity of a reaction under non-standard conditions by using the equation ΔG = ΔG° + RT ln Q, where Q is the reaction quotient.
What is the significance of Gibbs free energy in chemical reactions?
+The Gibbs free energy is a measure of the maximum amount of work that can be extracted from a system at constant temperature and pressure. It is used to predict the spontaneity of chemical reactions and determine the equilibrium constant of a reaction.
How is the standard Gibbs free energy change related to the equilibrium constant?
+The standard Gibbs free energy change is related to the equilibrium constant by the equation ΔG° = -RT ln K, where R is the gas constant and T is the temperature in Kelvin.
What are the limitations of Gibbs free energy calculations?
+Gibbs free energy calculations are limited by the accuracy of the enthalpy and entropy values used in the calculation. Additionally, the equation assumes that the reaction is at equilibrium, which may not always be the case.
In conclusion, Gibbs free energy calculations are a powerful tool for understanding the thermodynamics of chemical reactions. By combining the changes in enthalpy and entropy, we can determine the maximum amount of work that can be extracted from a system at constant temperature and pressure. The standard Gibbs free energy change is a measure of the spontaneity of a reaction under standard conditions, and the equation ΔG° = -RT ln K relates the standard Gibbs free energy change to the equilibrium constant. Accurate calculations of Gibbs free energy require precise values of enthalpy, entropy, and temperature, and the limitations of the equation must be considered when interpreting the results.