The calculation of molar mass is a fundamental concept in chemistry, crucial for determining the amount of a substance in a given sample. Molar mass, also known as molecular weight, is the total mass of a molecule of a substance, expressed in units of grams per mole (g/mol). It is calculated by summing the atomic masses of all the atoms in a molecule. The atomic masses of elements are found on the periodic table and are typically expressed in units of atomic mass units (amu) or grams per mole (g/mol).
Molar Mass Calculation
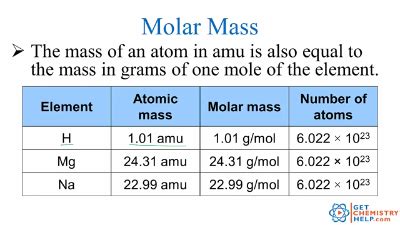
To calculate the molar mass of a compound, one must know the chemical formula of the compound and the atomic masses of its constituent elements. For example, to calculate the molar mass of sodium chloride (NaCl), we look up the atomic masses of sodium (Na) and chlorine (Cl) on the periodic table. The atomic mass of sodium is approximately 22.99 g/mol, and the atomic mass of chlorine is approximately 35.45 g/mol. The chemical formula NaCl indicates that one mole of sodium chloride contains one mole of sodium and one mole of chlorine. Therefore, the molar mass of NaCl is calculated as follows: Molar mass of NaCl = atomic mass of Na + atomic mass of Cl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol.
Step-by-Step Calculation Process
The step-by-step process for calculating the molar mass of a compound involves several key steps. First, write down the chemical formula of the compound. Next, look up the atomic masses of all the elements present in the compound from the periodic table. Then, multiply the atomic mass of each element by the number of atoms of that element in the compound’s chemical formula. Finally, add up all these products to obtain the total molar mass of the compound. For compounds with more complex formulas, such as those involving parentheses or subscripts, it is essential to apply the rules of arithmetic operations carefully, ensuring that the multiplication is performed before addition when calculating the total molar mass.
| Element | Atomic Mass (g/mol) | Number of Atoms | Total Mass (g/mol) |
|---|---|---|---|
| Sodium (Na) | 22.99 | 1 | 22.99 |
| Chlorine (Cl) | 35.45 | 1 | 35.45 |
| Total | - | - | 58.44 |
Understanding and accurately calculating molar mass is essential for various chemical calculations, including determining the number of moles of a substance, calculating the mass of a substance needed for a reaction, and understanding the stoichiometry of chemical reactions. By mastering the calculation of molar mass, chemists and researchers can perform a wide range of experiments and analyses with precision, contributing to advancements in fields such as materials science, pharmaceuticals, and environmental science.
Practical Applications and Considerations
In practical applications, the calculation of molar mass is not limited to simple compounds like NaCl. It is equally important for complex molecules, such as proteins, polymers, and pharmaceutical drugs, where knowing the exact molar mass can significantly impact the outcomes of experiments, the efficacy of drugs, and the properties of materials. Moreover, in industrial processes, the accurate calculation of molar mass is critical for ensuring the quality and consistency of products, managing resources efficiently, and minimizing waste.
Key Points
- The molar mass of a compound is calculated by summing the atomic masses of all the atoms in the molecule.
- Accurate calculation of molar mass requires the correct chemical formula and up-to-date atomic masses of the constituent elements.
- The calculation involves multiplying the atomic mass of each element by the number of atoms of that element in the formula and then adding these products together.
- Molar mass is crucial for various chemical calculations, including determining the number of moles of a substance and the stoichiometry of chemical reactions.
- Practical applications of molar mass calculation include materials science, pharmaceuticals, environmental science, and industrial processes.
The calculation of molar mass, while straightforward in concept, requires attention to detail and an understanding of the underlying principles of chemistry. By applying these principles and staying updated with the latest values for atomic masses, scientists and researchers can ensure the accuracy and reliability of their work, contributing to advancements in numerous fields of science and technology.
What is the primary purpose of calculating the molar mass of a compound?
+The primary purpose of calculating the molar mass of a compound is to determine the mass of one mole of the substance, which is essential for various chemical calculations, including the preparation of solutions, the determination of the number of moles of a substance, and the calculation of the stoichiometry of chemical reactions.
How does the molar mass of a compound relate to its chemical formula?
+The molar mass of a compound is directly related to its chemical formula, as the formula specifies the number of atoms of each element present in one molecule of the compound. By knowing the chemical formula, one can calculate the molar mass by summing the atomic masses of all the atoms in the molecule, taking into account the number of atoms of each element as indicated by the formula.
What are some common applications of molar mass calculations in everyday life?
+Molar mass calculations have numerous applications in everyday life, including the production of medicines, where the exact amount of active ingredients must be known; in the food industry, where the nutritional content of products is calculated based on the molar mass of ingredients; and in environmental science, where the impact of pollutants can be assessed based on their molar mass and concentration.