The H2O Lewis dot structure is a fundamental concept in chemistry, representing the molecular structure of water. To understand this, we first need to grasp what Lewis dot structures are. Developed by Gilbert N. Lewis, these structures are a way to represent the valence electrons in an atom, using dots to symbolize electrons. When it comes to water (H2O), its Lewis dot structure is crucial for understanding its properties and reactivity.
Naturally Worded Primary Topic Section with Semantic Relevance
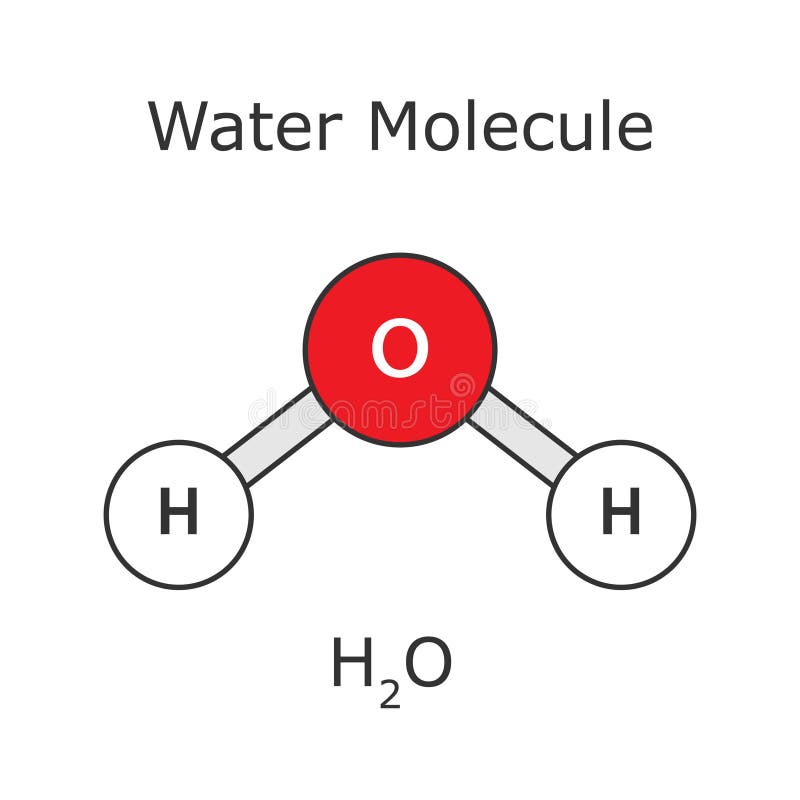
The water molecule consists of two hydrogen atoms bonded to a single oxygen atom. Oxygen, being in group 16 of the periodic table, has six valence electrons, while hydrogen, in group 1, has one valence electron. The Lewis dot structure for water shows the oxygen atom with four pairs of electrons (two lone pairs and two bonding pairs), and each hydrogen atom shares one pair of electrons with the oxygen, forming a covalent bond. This structure is vital because it illustrates the distribution of electrons, which in turn influences the molecule’s polarity and its ability to form hydrogen bonds.
Specific Subtopic with Natural Language Phrasing
The process of drawing the Lewis dot structure for H2O involves several steps. First, you determine the total number of valence electrons in the molecule. Oxygen has 6 valence electrons, and each hydrogen has 1, giving a total of 8 valence electrons (6 from oxygen + 2*1 from the two hydrogens). Next, you draw the skeletal structure, placing the atoms relative to each other. Since oxygen is more electronegative, it typically goes in the center, with the hydrogens bonded to it. Then, you distribute the electrons to satisfy the octet rule for each atom, which states that an atom will try to have eight electrons in its outermost shell to achieve stability. For water, this means the oxygen will have four pairs of electrons (two pairs are shared with the hydrogens, and two are lone pairs), and each hydrogen will share one pair with the oxygen.
| Atom | Valence Electrons | Lone Pairs | Bonding Pairs |
|---|---|---|---|
| Oxygen | 6 | 2 | 2 |
| Hydrogen | 1 | 0 | 1 |
Key Points
- The H2O Lewis dot structure represents the distribution of valence electrons in a water molecule, showing two hydrogen atoms bonded to an oxygen atom.
- Oxygen has six valence electrons and forms two covalent bonds with hydrogen atoms, leaving two lone pairs of electrons.
- The structure illustrates the molecule's polarity, with the oxygen end being partially negative and the hydrogen ends being partially positive, due to the difference in electronegativity between oxygen and hydrogen.
- The polarity of the water molecule allows it to form hydrogen bonds, which are crucial for many of water's unique properties and its role in biological systems.
- Understanding the Lewis dot structure of H2O is fundamental to grasping its chemical properties and behavior, including its role as a solvent and its participation in chemical reactions.
Advanced Aspects of H2O Lewis Dot Structure
One of the advanced aspects of the H2O Lewis dot structure is its implications for the physical and chemical properties of water. The bent or V-shape of the molecule, which arises from the two bonding pairs and two lone pairs of electrons around the oxygen atom, contributes to its high boiling point and surface tension. These properties, in turn, are essential for many of water’s roles in biological and environmental systems. Moreover, the Lewis structure helps in understanding the reactivity of water, including its ability to donate or accept electrons in redox reactions, which is pivotal in both biological processes and industrial applications.
Practical Applications and Real-World Examples
The practical applications of understanding the H2O Lewis dot structure are diverse and far-reaching. In biology, the structure of water is crucial for the folding of proteins, the stability of DNA, and the transport of nutrients and waste products across cell membranes. In chemistry, the polarity of water makes it an excellent solvent for ionic compounds, which is vital for many chemical reactions and processes. Additionally, the unique properties of water, such as its high specific heat capacity and latent heat of vaporization, play critical roles in regulating Earth’s climate and weather patterns.
| Property | Value | Importance |
|---|---|---|
| Boiling Point | 100°C at 1 atm | High boiling point contributes to water's role in climate regulation |
| Surface Tension | 72 mN/m at 20°C | Allows water to resist external forces, crucial for many biological processes |
What is the significance of the Lewis dot structure for H2O in understanding its chemical properties?
+The Lewis dot structure for H2O is significant because it illustrates the distribution of electrons within the molecule, which influences its polarity, reactivity, and ability to form hydrogen bonds. These properties are essential for water's role as a solvent, its participation in chemical reactions, and its unique physical properties.
How does the polarity of the water molecule, as indicated by its Lewis dot structure, affect its physical properties?
+The polarity of the water molecule contributes to its high boiling point, surface tension, and specific heat capacity. These properties are critical for water's roles in biological systems, climate regulation, and as a medium for chemical reactions.
What are some real-world applications of understanding the H2O Lewis dot structure?
+Understanding the H2O Lewis dot structure has applications in fields such as biology (protein folding, DNA stability), chemistry (solvent properties, reaction mechanisms), and environmental science (climate regulation, water cycle processes). It's fundamental for appreciating water's unique role in supporting life and regulating Earth's systems.
In conclusion, the H2O Lewis dot structure provides a fundamental understanding of the water molecule’s electronic distribution, polarity, and chemical behavior. Its significance extends beyond the realm of chemistry to biology, environmental science, and everyday life, highlighting the importance of basic chemical principles in understanding complex phenomena. By grasping the concepts represented by the Lewis dot structure of H2O, we can better appreciate the intricate roles water plays in our world and the importance of continued research into its properties and behaviors.