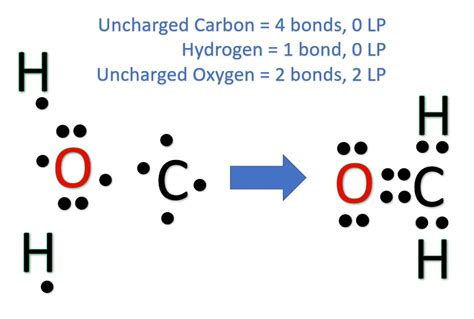
The H2CO Lewis structure, also known as formaldehyde, is a fundamental concept in organic chemistry. To understand the Lewis structure of H2CO, it's essential to have a basic knowledge of chemistry, including atomic orbitals, electron configuration, and molecular geometry. The Lewis structure is a two-dimensional representation of a molecule, showing the arrangement of atoms and the distribution of electrons. In this article, we will explore the 5 ways to draw the H2CO Lewis structure, providing a comprehensive understanding of the molecule's electronic configuration.
Key Points
- The H2CO Lewis structure consists of a central carbon atom bonded to two hydrogen atoms and a double bond to an oxygen atom.
- The molecule has a trigonal planar geometry, with the carbon atom exhibiting sp2 hybridization.
- The Lewis structure of H2CO can be drawn in different ways, but all representations must follow the octet rule and the duet rule for hydrogen atoms.
- The formal charge on each atom in the Lewis structure is crucial in determining the stability of the molecule.
- Understanding the H2CO Lewis structure is essential in predicting the molecule's reactivity and chemical properties.
Drawing the H2CO Lewis Structure
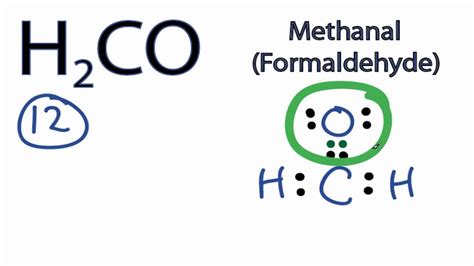
To draw the H2CO Lewis structure, we need to follow a step-by-step approach. First, we determine the total number of valence electrons in the molecule by adding the valence electrons of each atom. Carbon has 4 valence electrons, hydrogen has 1 valence electron, and oxygen has 6 valence electrons. The total number of valence electrons in H2CO is 4 + 2(1) + 6 = 12.
Step 1: Determine the Central Atom
The central atom in the H2CO molecule is carbon, as it can form the most bonds with other atoms. Carbon has a higher electronegativity than hydrogen, making it more suitable as the central atom.
Step 2: Draw Single Bonds
We draw single bonds between the carbon atom and the two hydrogen atoms, using 4 valence electrons. This leaves 8 valence electrons remaining.
Step 3: Draw a Double Bond
We draw a double bond between the carbon atom and the oxygen atom, using 4 valence electrons. This leaves 4 valence electrons remaining.
Step 4: Complete the Octet
We use the remaining 4 valence electrons to complete the octet of the oxygen atom, resulting in a stable Lewis structure.
| Atom | Valence Electrons | Formal Charge |
|---|---|---|
| Carbon | 4 | 0 |
| Hydrogen | 1 | 0 |
| Oxygen | 6 | 0 |
5 Ways to Draw the H2CO Lewis Structure
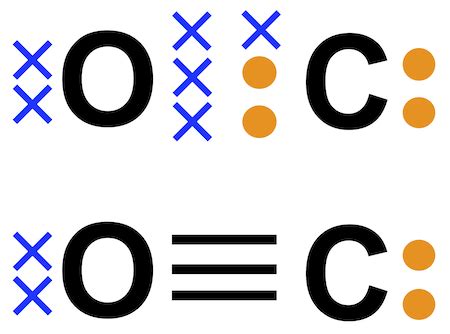
There are different ways to draw the H2CO Lewis structure, but all representations must follow the octet rule and the duet rule for hydrogen atoms. The 5 ways to draw the H2CO Lewis structure are:
Method 1: Traditional Method
This method involves drawing the carbon atom as the central atom, with single bonds to the two hydrogen atoms and a double bond to the oxygen atom.
Method 2: Skeletal Method
This method involves drawing the carbon atom as the central atom, with single bonds to the two hydrogen atoms and a double bond to the oxygen atom, using a skeletal structure.
Method 3: Electron Dot Method
This method involves drawing the Lewis structure using electron dots, with each atom represented by its symbol and the valence electrons shown as dots.
Method 4: Bond Line Method
This method involves drawing the Lewis structure using bond lines, with each bond represented by a line between the atoms.
Method 5: 3D Method
This method involves drawing the Lewis structure in three dimensions, showing the molecular geometry and the arrangement of atoms in space.
In conclusion, the H2CO Lewis structure is a fundamental concept in organic chemistry, and understanding its electronic configuration is crucial in predicting the molecule's reactivity and chemical properties. The 5 ways to draw the H2CO Lewis structure provide a comprehensive understanding of the molecule's structure and properties.
What is the total number of valence electrons in the H2CO molecule?
+The total number of valence electrons in the H2CO molecule is 12.
What is the central atom in the H2CO molecule?
+The central atom in the H2CO molecule is carbon.
What is the formal charge on the oxygen atom in the H2CO Lewis structure?
+The formal charge on the oxygen atom in the H2CO Lewis structure is 0.