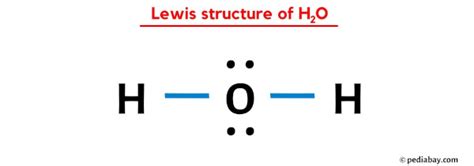
The Lewis dot structure is a fundamental concept in chemistry that helps visualize the arrangement of electrons in atoms and molecules. When it comes to water, or H2O, understanding its Lewis dot structure is crucial for grasping its chemical properties and behavior. Water is composed of two hydrogen atoms and one oxygen atom, and its molecular structure can be represented using Lewis dots.
Naturally Worded Primary Topic Section with Semantic Relevance
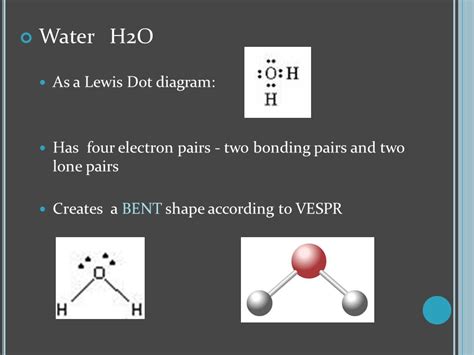
To draw the Lewis dot structure of H2O, we start by determining the total number of valence electrons available. Oxygen, being in group 16 of the periodic table, has 6 valence electrons, while each hydrogen atom, found in group 1, contributes 1 valence electron. Therefore, the total number of valence electrons in a water molecule is 2 (from the two hydrogen atoms) + 6 (from the oxygen atom) = 8 electrons. The next step involves arranging these electrons to satisfy the octet rule for each atom, which states that an atom tends to gain, lose, or share electrons to achieve a full outer shell with 8 electrons, resembling the noble gas configuration.
Specific Subtopic with Natural Language Phrasing
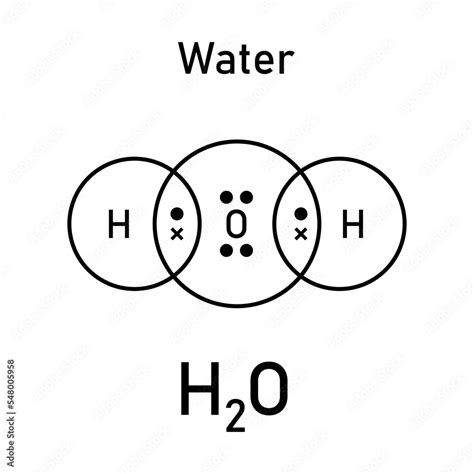
The oxygen atom, having 6 valence electrons, needs 2 more electrons to achieve the octet configuration. Each hydrogen atom, with its single valence electron, seeks one additional electron to fulfill its duet (2-electron) requirement. By sharing electrons, the oxygen atom forms two covalent bonds with the hydrogen atoms. In the Lewis dot structure, the oxygen atom is placed at the center, and the two hydrogen atoms are positioned on either side. The shared electrons are represented by a line between the atoms, and any remaining electrons are drawn as dots around the atoms. For H2O, the oxygen atom shares 4 electrons with the hydrogen atoms (2 electrons in each covalent bond) and has 4 electrons remaining, which are depicted as two pairs of dots on the oxygen atom.
| Atom | Valence Electrons | Shared Electrons | Unshared Electrons |
|---|---|---|---|
| Oxygen (O) | 6 | 4 | 4 |
| Hydrogen (H) | 1 | 2 (1 from each H) | 0 |
Key Points
- The Lewis dot structure of H2O is drawn by placing the oxygen atom at the center and arranging the hydrogen atoms on either side, forming two covalent bonds.
- Oxygen has 6 valence electrons and shares 4 of them with the hydrogen atoms, leaving 4 electrons unshared.
- Each hydrogen atom shares 2 electrons with the oxygen atom, fulfilling its duet requirement.
- The Lewis dot structure helps explain the polarity of the water molecule, which arises from the unequal sharing of electrons between oxygen and hydrogen.
- Understanding the molecular structure of H2O is essential for grasping its chemical properties and behavior in various reactions and biological processes.
Technical Specifications and Contextual Explanation
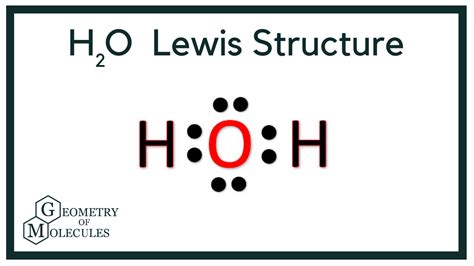
The polarity of water, as inferred from its Lewis dot structure, plays a significant role in its chemical and biological properties. Water’s ability to form hydrogen bonds, which are relatively weak electrostatic attractions between the partial positive charge on a hydrogen atom of one molecule and the partial negative charge on an oxygen atom of another molecule, is a direct consequence of its polarity. This property is vital for the solubility of substances in water and for many biological processes, including protein folding and cell membrane structure.
Evidence-Based Analysis with Balanced Perspective
While the Lewis dot structure provides a simplified view of the electron arrangement in H2O, it is essential to recognize its limitations. The structure does not account for the actual shape of the molecule or the distribution of electron density. However, when combined with other theoretical models, such as VSEPR (Valence Shell Electron Pair Repulsion) theory, it offers a more comprehensive understanding of the molecular geometry and chemical reactivity of water. The VSEPR model predicts that the water molecule has a bent or V-shape, which is consistent with experimental observations and further supports the concept of polarity in H2O.
In conclusion, the Lewis dot structure of H2O is a fundamental tool for understanding the chemical properties and behavior of water. By recognizing the arrangement of electrons in the molecule and the resulting polarity, we can better comprehend the unique characteristics of water that make it essential for life and chemical processes.
What is the total number of valence electrons in a water molecule?
+The total number of valence electrons in a water molecule (H2O) is 8, which includes 6 valence electrons from the oxygen atom and 1 valence electron from each of the two hydrogen atoms.
Why is the Lewis dot structure important for understanding the polarity of H2O?
+The Lewis dot structure helps visualize the unequal sharing of electrons between the oxygen and hydrogen atoms, which leads to the polarity of the water molecule. This polarity is crucial for many of water's chemical and biological properties.
How does the polarity of water influence its ability to form hydrogen bonds?
+The polarity of water, with partial positive charges on the hydrogen atoms and a partial negative charge on the oxygen atom, allows it to form hydrogen bonds. These are weak electrostatic attractions between the partial positive charge on a hydrogen atom of one molecule and the partial negative charge on an oxygen atom of another molecule, which are essential for the solubility of substances in water and many biological processes.
Meta Description: Understand the Lewis dot structure of H2O, including its polarity and how it influences the chemical and biological properties of water, essential for grasping its role in life and chemical processes.