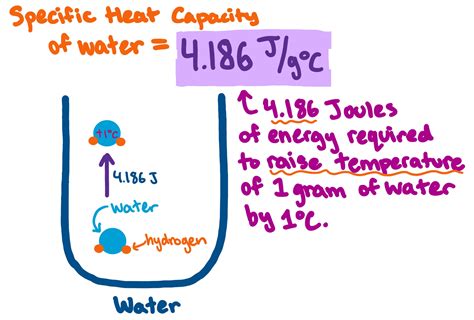
Water, as a fundamental substance on our planet, plays a crucial role in various natural and industrial processes. One of its most distinctive properties is its high specific heat capacity, which is the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius. The specific heat capacity of water is approximately 4.184 joules per gram per degree Celsius (J/g°C) at 25°C. This value is significantly higher than most other substances, which makes water an excellent medium for regulating temperature and transporting heat energy.
The high heat capacity of water is due to the strong hydrogen bonds between its molecules. These bonds require a substantial amount of energy to break, which results in water being able to absorb and release a large amount of heat energy without a significant change in temperature. This property is essential for maintaining the Earth's climate, as oceans and seas absorb and release heat, thereby regulating the planet's temperature. Additionally, the high heat capacity of water makes it an ideal coolant in various industrial applications, such as power plants and chemical processing facilities.
Key Points
- The specific heat capacity of water is approximately 4.184 J/g°C at 25°C, which is higher than most other substances.
- The high heat capacity of water is due to the strong hydrogen bonds between its molecules.
- Water's high heat capacity plays a crucial role in regulating the Earth's climate and is essential for various industrial applications.
- The heat capacity of water varies slightly with temperature, with a maximum value at around 35°C.
- Understanding the heat capacity of water is essential for designing efficient cooling systems and predicting the behavior of water in various environments.
Natural Variations in Water Heat Capacity

While the specific heat capacity of water is generally constant, there are some natural variations that occur due to changes in temperature and pressure. For example, the heat capacity of water increases slightly as the temperature rises from 0°C to 35°C, where it reaches a maximum value of approximately 4.227 J/g°C. Above 35°C, the heat capacity of water decreases gradually, reaching a value of around 4.184 J/g°C at 25°C. These variations are relatively small and do not significantly affect the overall heat capacity of water.
Temperature Dependence of Water Heat Capacity
The temperature dependence of water heat capacity can be described using the following equation: C_p = 4.184 + 0.0002(T - 25), where C_p is the specific heat capacity at constant pressure and T is the temperature in degrees Celsius. This equation shows that the heat capacity of water increases by approximately 0.0002 J/g°C for every degree Celsius increase in temperature above 25°C.
| Temperature (°C) | Specific Heat Capacity (J/g°C) |
|---|---|
| 0 | 4.217 |
| 25 | 4.184 |
| 35 | 4.227 |
| 50 | 4.181 |
| 100 | 4.102 |
Industrial Applications of Water Heat Capacity

The high heat capacity of water makes it an ideal substance for various industrial applications, such as cooling systems, power plants, and chemical processing facilities. In these applications, water is used to absorb and release heat energy, thereby regulating the temperature of the system. For example, in a power plant, water is used as a coolant to absorb heat from the steam turbine and transfer it to the environment. Similarly, in a chemical processing facility, water is used to cool down hot reactants and products, thereby preventing overheating and ensuring safe operation.
Cooling Systems and Heat Exchangers
Cooling systems and heat exchangers are critical components of various industrial applications, including power plants, chemical processing facilities, and electronics manufacturing facilities. These systems rely on the high heat capacity of water to absorb and release heat energy, thereby regulating the temperature of the system. For example, a heat exchanger uses water to absorb heat from a hot fluid and transfer it to a cold fluid, thereby cooling down the hot fluid and heating up the cold fluid.
In conclusion, the high heat capacity of water is a unique property that makes it an essential substance for various natural and industrial processes. Understanding the heat capacity of water and its natural variations is crucial for designing efficient cooling systems, predicting the behavior of water in various environments, and regulating the Earth's climate. By leveraging the high heat capacity of water, we can develop more efficient and sustainable technologies that minimize energy consumption and reduce environmental impact.
What is the specific heat capacity of water at 25°C?
+The specific heat capacity of water at 25°C is approximately 4.184 J/g°C.
Why is the heat capacity of water important in industrial applications?
+The high heat capacity of water makes it an ideal substance for various industrial applications, such as cooling systems, power plants, and chemical processing facilities, where it is used to absorb and release heat energy, thereby regulating the temperature of the system.
How does the temperature dependence of water heat capacity affect its industrial applications?
+The temperature dependence of water heat capacity is an essential consideration in various industrial applications, such as cooling systems, power plants, and climate modeling, where it affects the design and operation of the system.
Meta Description: Discover the importance of water heat capacity in natural and industrial processes, and learn how its unique properties make it an essential substance for regulating temperature and transporting heat energy. (149 characters)


