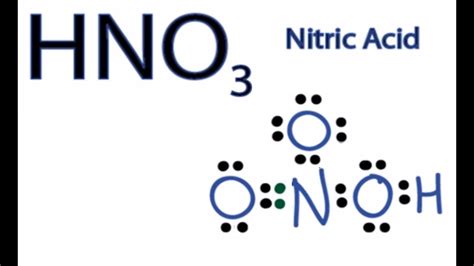
Nitric acid, denoted by the chemical formula HNO3, is a highly corrosive and toxic strong acid that plays a crucial role in various industrial and laboratory processes. Its strength as an acid is attributed to several key factors, which are fundamental to understanding its chemical properties and behaviors. In this article, we will delve into the reasons behind the strength of HNO3, exploring its chemical structure, dissociation characteristics, and the implications of its acidity in different contexts.
Chemical Structure and Dissociation

The chemical structure of HNO3 consists of a hydrogen atom bonded to a nitrate ion (NO3-), which is a polyatomic ion composed of one nitrogen atom and three oxygen atoms. This structure facilitates the easy dissociation of HNO3 in water, leading to the formation of hydronium ions (H3O+) and nitrate ions (NO3-). The ease with which HNO3 dissociates is a primary indicator of its strength as an acid. According to the Arrhenius theory, a strong acid is one that completely dissociates in water, and HNO3 fits this definition well, with a dissociation constant (Ka) of approximately 24 at 25°C.
Dissociation Constant and Acid Strength
The dissociation constant (Ka) is a quantitative measure of the strength of an acid in solution. For HNO3, the dissociation reaction can be represented as HNO3 + H2O → H3O+ + NO3-. The Ka value for this reaction is significantly high, indicating that HNO3 is almost completely ionized in aqueous solutions. This high degree of dissociation is what classifies HNO3 as a strong acid, distinguishing it from weak acids like acetic acid (CH3COOH), which has a much lower Ka value and does not dissociate as completely in water.
| Acid | Ka Value at 25°C |
|---|---|
| HNO3 (Nitric Acid) | 24 |
| CH3COOH (Acetic Acid) | 1.8 x 10^-5 |

Electronegativity and Bond Strength
Another factor contributing to the strength of HNO3 is the electronegativity of the atoms involved in its structure, particularly the oxygen and nitrogen atoms. The high electronegativity of these atoms pulls electrons away from the hydrogen atom, weakening the O-H or N-H bond and making it easier for the hydrogen ion (H+) to dissociate. This effect is more pronounced in HNO3 due to the presence of three oxygen atoms, each exerting an electronegative pull, which collectively enhances the acid’s dissociation and thus its strength.
Resonance Stabilization of the Conjugate Base
The strength of HNO3 is also influenced by the stability of its conjugate base, NO3-. The nitrate ion is stabilized by resonance, a phenomenon where the arrangement of electrons in the molecule can be described by multiple Lewis structures. This resonance stabilization distributes the negative charge across the three oxygen atoms, reducing the charge density on any single atom and thus stabilizing the ion. A stable conjugate base is indicative of a strong acid, as it implies that the acid can readily donate a proton without significant energetic penalty.
Key Points
- HNO3's complete dissociation in water is a hallmark of its strength as an acid.
- The high Ka value of HNO3 indicates its strong acidic nature compared to weak acids.
- Electronegativity of oxygen and nitrogen atoms in HNO3 facilitates the dissociation of the hydrogen ion.
- Resonance stabilization of the nitrate ion (NO3-) contributes to the strength of HNO3 by making its conjugate base more stable.
- The chemical structure and properties of HNO3 make it a crucial component in various industrial and laboratory applications.
Industrial and Laboratory Applications
The strength of HNO3 has significant implications for its use in industrial processes and laboratory settings. It is commonly used in the production of fertilizers, explosives, and in the process of etching and cleaning metal surfaces. In laboratories, HNO3 serves as a strong acid in various titrations and is used to prepare other acids and compounds. Its ability to donate a proton readily makes it an essential reagent in organic synthesis and analytical chemistry.
Safety and Handling Considerations
Given its corrosive nature and toxicity, handling HNO3 requires careful attention to safety protocols. It can cause severe burns upon contact with skin and eyes and can release toxic fumes when inhaled. Thus, when working with HNO3, it is crucial to wear appropriate protective gear, including gloves, goggles, and a mask, and to work in a well-ventilated area or fume hood.
What are the primary uses of HNO3 in industry?
+HNO3 is primarily used in the production of fertilizers, such as ammonium nitrate, and in the manufacture of explosives. It is also used for etching and cleaning metal surfaces.
Why is HNO3 considered a strong acid?
+HNO3 is considered a strong acid because it completely dissociates in water, producing hydronium ions (H3O+) and nitrate ions (NO3-), and has a high dissociation constant (Ka).
What precautions should be taken when handling HNO3?
+When handling HNO3, one should wear protective gear including gloves, goggles, and a mask, and work in a well-ventilated area or fume hood to avoid exposure to toxic fumes and skin/eye contact.
In conclusion, the strength of HNO3 can be attributed to its chemical structure, high dissociation constant, electronegativity of its constituent atoms, and the resonance stabilization of its conjugate base. These factors not only underscore its potency as a strong acid but also highlight its versatility and importance in various industrial and laboratory applications. Understanding the properties and behaviors of HNO3 is crucial for harnessing its potential while ensuring safe handling and use.