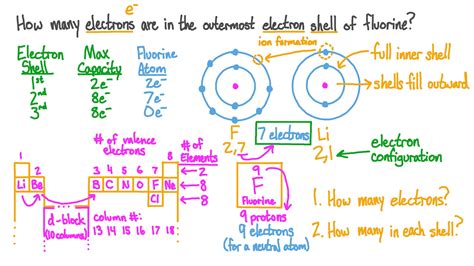
Understanding the behavior and location of electrons is fundamental in various fields of science and technology, including physics, chemistry, and engineering. Electrons, being the negatively charged particles that orbit the nucleus of an atom, play a crucial role in determining the chemical properties of elements and compounds. Finding electrons or understanding their distribution within an atom or molecule is essential for predicting chemical reactivity, understanding electrical conductivity, and designing electronic devices. Here, we will explore five ways to find or understand the distribution of electrons in atoms and molecules.
Naturally worded primary topic section with semantic relevance
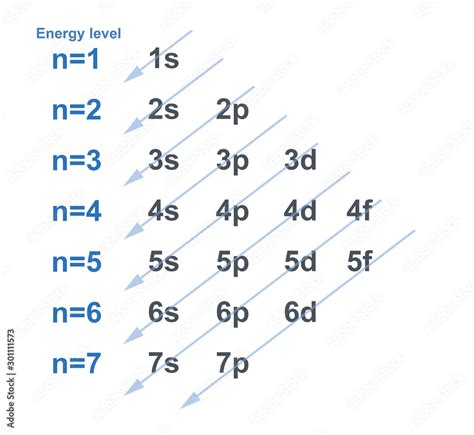
The first method involves the use of quantum mechanics, specifically the Schrödinger equation, to predict the probability of finding an electron within a certain region around the nucleus. This method provides a theoretical framework for understanding electron distribution by solving for the wave functions of electrons in different energy states. The resulting probability distributions, or electron clouds, give a detailed picture of where electrons are likely to be found. This approach is fundamental in atomic physics and chemistry, allowing for the calculation of electron configurations and the prediction of chemical properties.
Specific subtopic with natural language phrasing
A second approach to finding electrons involves experimental techniques such as electron spectroscopy. Electron spectroscopy encompasses various methods, including photoelectron spectroscopy (PES) and Auger electron spectroscopy (AES), which measure the energy of electrons emitted from a material when it is exposed to high-energy radiation, such as ultraviolet light or X-rays. By analyzing the energies of the emitted electrons, scientists can infer the binding energies of electrons in different orbitals, providing insights into the electronic structure of the material. This information is crucial for understanding the chemical and physical properties of materials at the atomic and molecular level.
| Technique | Description | Application |
|---|---|---|
| Photoelectron Spectroscopy (PES) | Measures the energy of electrons emitted when a material is irradiated with light | Understanding valence band structures in solids |
| Auger Electron Spectroscopy (AES) | Analyzes the energy of electrons emitted after the creation of a core hole | Surface analysis and composition determination |
| X-ray Photoelectron Spectroscopy (XPS) | Measures the energy of electrons emitted when a material is irradiated with X-rays | Chemical composition and electronic state analysis of surfaces |
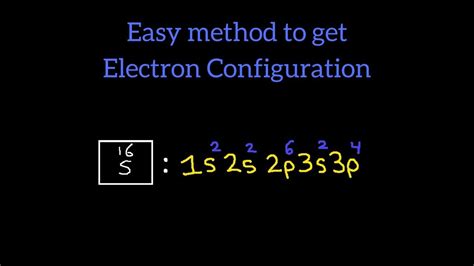
Key Points
- Quantum mechanics provides a theoretical framework for predicting electron distribution through the solution of the Schrödinger equation.
- Electron spectroscopy techniques, such as PES and AES, offer experimental means to study electron energies and configurations.
- Understanding electron distribution is essential for predicting chemical reactivity and designing electronic devices.
- Theoretical models, such as the atomic orbital model, simplify the understanding of electron configuration in atoms.
- Experimental techniques like X-ray diffraction can indirectly inform about electron distribution by determining the structure of molecules and crystals.
A third method for finding electrons involves the use of theoretical models, such as the atomic orbital model. This model simplifies the understanding of electron configuration by describing the distribution of electrons in an atom in terms of orbitals, which are mathematical functions that describe the wave-like behavior of electrons. The atomic orbital model is a fundamental tool in chemistry, allowing for the prediction of chemical properties and reactivity based on the arrangement of electrons in atoms and molecules.
Advanced Experimental Techniques
A fourth approach to understanding electron distribution is through advanced experimental techniques such as scanning tunneling microscopy (STM) and scanning transmission electron microscopy (STEM). STM allows for the direct observation of electron density on surfaces at the atomic scale by measuring the tunneling current between a sharp tip and the surface. STEM, on the other hand, uses a beam of electrons to produce high-resolution images of the internal structure of materials, providing insights into the arrangement of atoms and, indirectly, the distribution of electrons within the material.
Computational Methods
A fifth method involves computational simulations, such as density functional theory (DFT) calculations. DFT is a computational quantum mechanical modeling method used to predict the electronic and atomic structure of many-body systems. It provides a powerful tool for understanding electron distribution in complex systems, including molecules, solids, and surfaces. By solving for the electron density and energy levels of a system, DFT calculations can predict properties such as chemical reactivity, electrical conductivity, and optical properties, making it an indispensable tool in materials science and chemistry research.
What is the significance of understanding electron distribution in atoms and molecules?
+Understanding electron distribution is crucial for predicting chemical properties, reactivity, and designing electronic devices. It underpins our ability to develop new materials and technologies.
How do experimental techniques like electron spectroscopy contribute to our understanding of electrons?
+Electron spectroscopy techniques provide direct evidence of electron energies and configurations, allowing for the validation of theoretical models and a deeper understanding of material properties.
What role do computational methods play in the study of electron distribution?
+Computational methods, such as DFT calculations, offer a powerful means to predict and understand electron distribution in complex systems, complementing experimental techniques and theoretical models.
In conclusion, understanding electron distribution is a multifaceted endeavor that draws on theoretical models, experimental techniques, and computational simulations. Each approach provides unique insights into the behavior and distribution of electrons in atoms and molecules, underpinning advancements in fields from chemistry and materials science to electronics and engineering. As research continues to refine our understanding of electron distribution, we can expect further innovations in technology and deeper insights into the fundamental nature of matter.