To find the number of electrons in an atom, you need to understand the basic structure of atoms and how electrons are arranged. Atoms are the building blocks of matter, and they consist of three main parts: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, which is the center of the atom, while electrons orbit around the nucleus in electron shells or energy levels.
Understanding Atomic Structure
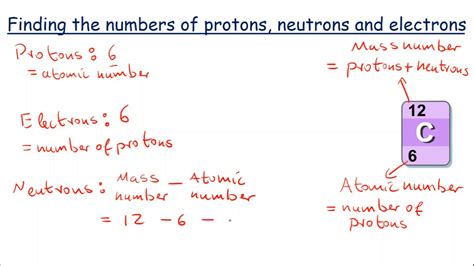
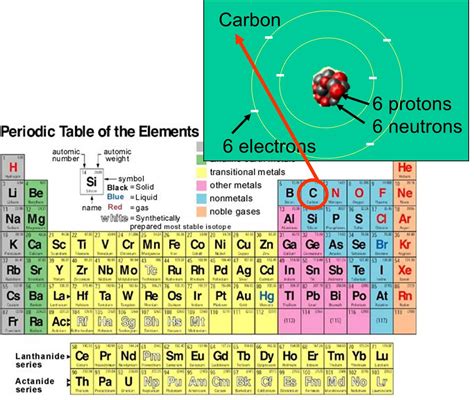
The atomic number of an element, which is the number of protons in the nucleus of an atom, is crucial for determining the number of electrons. In a neutral atom, the number of electrons is equal to the number of protons. This balance is what makes the atom electrically neutral, as protons have a positive charge and electrons have a negative charge. The number of neutrons can vary, leading to different isotopes of the same element, but it does not affect the number of electrons in a neutral atom.
Calculating the Number of Electrons
To calculate the number of electrons in an atom, follow these steps:
- Identify the element: Determine the element you are working with by its name or symbol.
- Find the atomic number: Look up the atomic number of the element. This can be found on the periodic table. The atomic number is the number of protons in the nucleus and, in a neutral atom, equals the number of electrons.
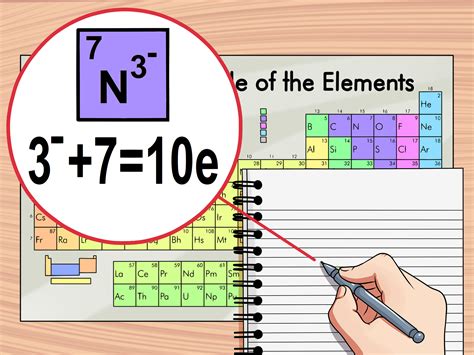
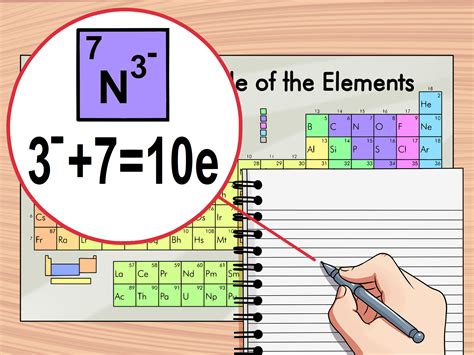
- Determine the charge (if applicable): If the atom is an ion, you need to know its charge. The charge is the difference between the number of protons and electrons. For a positively charged ion (cation), electrons have been removed, while for a negatively charged ion (anion), electrons have been added.
- Calculate the number of electrons: If the atom is neutral, the number of electrons equals the atomic number. If it’s an ion, adjust the atomic number by the charge to find the number of electrons. For example, if it’s a +2 ion, subtract 2 from the atomic number. If it’s a -2 ion, add 2 to the atomic number.
| Element | Atomic Number | Number of Electrons (Neutral) |
|---|---|---|
| Hydrogen (H) | 1 | 1 |
| Helium (He) | 2 | 2 |
| Oxygen (O) | 8 | 8 |
Key Points
- The number of electrons in a neutral atom equals the atomic number.
- For ions, the number of electrons is adjusted by the charge: add electrons for negative ions, subtract for positive ions.
- The atomic number can be found on the periodic table and represents the number of protons in the nucleus.
- Understanding the difference between atomic number, mass number, and charge is crucial for calculating the number of electrons.
- Neutral atoms have an equal number of protons and electrons, making them electrically neutral.
Practical Applications
Understanding how to find the number of electrons is essential in chemistry and physics. It helps in calculating the electronic configuration of atoms, which is vital for understanding chemical bonding, reactivity, and the physical properties of elements. In ionic bonding, for instance, electrons are transferred between atoms, resulting in the formation of ions that then attract each other. Knowing the number of electrons in each atom involved in a chemical reaction can help predict the products and the conditions required for the reaction to occur.
Electronic Configuration
The arrangement of electrons in an atom, or its electronic configuration, follows specific rules and is crucial for understanding chemical properties. The electrons fill the energy levels or shells around the nucleus in a particular order, following the Aufbau principle and the Pauli exclusion principle. This configuration dictates how atoms will interact with each other, forming bonds and creating compounds.
How do I find the number of electrons in an ion?
+To find the number of electrons in an ion, you start with the atomic number of the element (which gives the number of electrons in the neutral atom) and then adjust for the charge. If the ion has a positive charge, you subtract the charge from the atomic number. If the ion has a negative charge, you add the charge to the atomic number.
What is the difference between atomic number and mass number?
+The atomic number is the number of protons in an atom's nucleus and determines the element's identity in the periodic table. The mass number, on the other hand, is the sum of protons and neutrons in the nucleus. It essentially gives the mass of the atom. The number of neutrons can vary, leading to different isotopes of the same element, which have the same atomic number (and thus the same number of electrons in a neutral state) but different mass numbers.
How does the number of electrons affect an atom's reactivity?
+The number of electrons, particularly the electrons in the outermost shell (valence electrons), plays a crucial role in determining an atom's reactivity. Atoms tend to react in ways that result in a full outer shell, which is a stable configuration. The number of valence electrons an atom has determines the types of chemical bonds it can form and with how many other atoms it can bond.
Understanding the basics of atomic structure and how to calculate the number of electrons is foundational in chemistry and physics. It sets the stage for more advanced topics, such as chemical bonding, reactions, and the properties of materials. By mastering these principles, individuals can better appreciate the complexities of the physical world and how elements interact at the atomic level.