Carbon, the sixth element in the periodic table, is a fundamental component of all living organisms and a cornerstone of organic chemistry. Its unique ability to form four bonds with other atoms, a property known as tetravalency, underlies the vast diversity of carbon-based compounds. This singular characteristic of carbon allows it to create complex molecules with a wide range of properties, from the simplicity of methane (CH4) to the intricacy of DNA and proteins. Understanding how carbon forms four bonds is crucial for grasping the basics of organic chemistry and the molecular basis of life.
Chemical Basis of Carbon’s Tetravalency
The chemical basis for carbon’s ability to form four bonds lies in its electronic configuration. Carbon has six electrons, with two in the inner shell and four in the outer shell. According to the octet rule, atoms tend to gain, lose, or share electrons to achieve a full outer shell, which typically contains eight electrons. Carbon, with its four valence electrons, can achieve this stable configuration by forming four covalent bonds with other atoms, each bond representing a shared pair of electrons. This is most commonly observed in the formation of tetrahedral molecules, where carbon is at the center, bonded to four other atoms or groups, with the bonds arranged in a three-dimensional tetrahedral geometry.
Types of Bonds Formed by Carbon
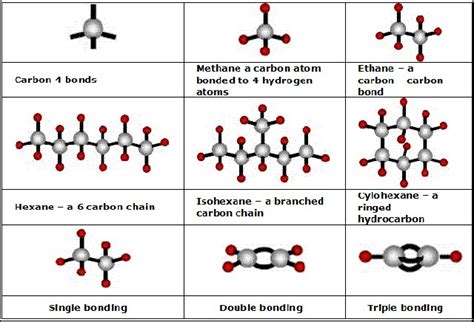
Carbon’s versatility in forming compounds is further enhanced by its ability to form different types of bonds, including sigma (σ) bonds and pi (π) bonds. Sigma bonds are formed by end-to-end overlap of atomic orbitals and are typically involved in the formation of single bonds. Pi bonds, on the other hand, are formed by side-by-side overlap of parallel p orbitals and are involved in the formation of double and triple bonds. This capacity to form various bond types allows carbon to participate in a wide range of chemical reactions and to form molecules with diverse structures and properties.
| Type of Bond | Description |
|---|---|
| Sigma (σ) Bond | Formed by end-to-end overlap of atomic orbitals, typically involved in single bonds. |
| Pi (π) Bond | Formed by side-by-side overlap of parallel p orbitals, involved in double and triple bonds. |
Biological and Industrial Significance
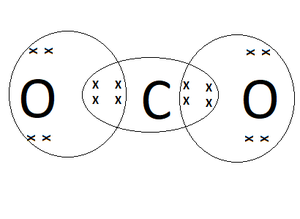
The ability of carbon to form four bonds is not only the foundation of organic chemistry but also underlies the complexity and diversity of biological molecules. In living organisms, carbon is a key component of biomolecules such as carbohydrates, lipids, proteins, and nucleic acids. The diversity of these biomolecules, which is essential for the complexity of life, arises from the variety of ways in which carbon can bond with other elements, particularly hydrogen, oxygen, nitrogen, and sulfur. Industrially, the versatility of carbon in forming compounds is exploited in the production of plastics, fuels, pharmaceuticals, and many other products that are crucial to modern society.
Environmental Considerations
While carbon’s ability to form four bonds is a boon for the diversity of life and industrial applications, it also has environmental implications. The burning of carbon-based fossil fuels, for example, releases carbon dioxide (CO2) into the atmosphere, contributing to climate change. Understanding the chemistry of carbon and its compounds is essential for developing strategies to mitigate these effects, such as the capture and utilization of CO2, and the development of sustainable, carbon-neutral energy sources.
Key Points
- Carbon's ability to form four bonds is due to its electronic configuration and the tendency to achieve a stable octet of electrons in its outer shell.
- The formation of sigma and pi bonds allows carbon to participate in a wide range of chemical reactions and to form molecules with diverse structures and properties.
- The versatility of carbon in forming compounds underlies the complexity and diversity of biological molecules and is crucial for industrial applications.
- Understanding carbon chemistry is essential for addressing environmental challenges, such as climate change, related to carbon-based fossil fuels.
- The development of sustainable technologies that utilize carbon efficiently and minimize environmental impact is a key area of research and development.
As research continues to uncover the intricacies of carbon chemistry and its applications, the importance of understanding how carbon forms four bonds remains at the forefront of scientific inquiry. This fundamental aspect of carbon's chemistry not only underpins the vast diversity of organic compounds but also holds the key to developing innovative solutions to environmental challenges and to advancing our understanding of the molecular basis of life.
What is the electronic basis for carbon’s ability to form four bonds?
+Carbon’s ability to form four bonds is based on its electronic configuration, where it has four valence electrons. According to the octet rule, carbon achieves a stable configuration by forming four covalent bonds with other atoms, each bond representing a shared pair of electrons.
What types of bonds can carbon form, and what are their characteristics?
+Carbon can form sigma (σ) bonds, which are involved in single bonds and formed by end-to-end overlap of atomic orbitals, and pi (π) bonds, which are involved in double and triple bonds and formed by side-by-side overlap of parallel p orbitals.
What is the significance of carbon’s ability to form four bonds in biological and industrial contexts?
+In biological contexts, carbon’s ability to form four bonds underlies the diversity and complexity of biomolecules essential for life. Industrially, this property is exploited in the production of a wide range of products, from plastics and fuels to pharmaceuticals.



