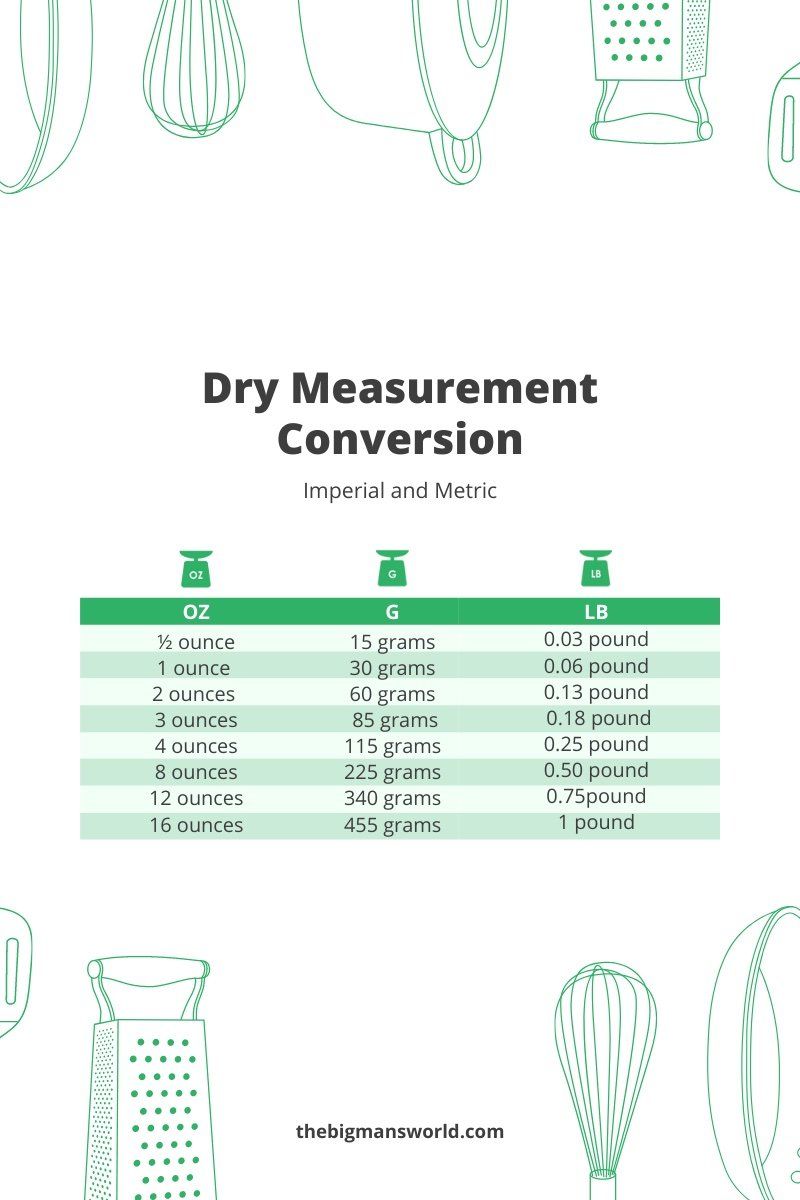
The relationship between fluid ounces and pounds is a fundamental concept in both cooking and chemistry, as it often requires the conversion between volume and weight measurements. Understanding this relationship is crucial for achieving accurate results in recipes and experimental procedures. In the United States, a common system of measurement is used, where 1 pound is equivalent to 16 ounces in terms of weight. However, when discussing fluid ounces, we are referring to a unit of volume. The conversion between fluid ounces and pounds depends on the density of the substance being measured, as 1 pound of different materials will occupy different volumes due to their varying densities.
For water, 1 pound is approximately equal to 16 fluid ounces, because the density of water is close to 1 gram per milliliter (g/mL), and there are 453.592 grams in a pound. Since 1 fluid ounce of water weighs approximately 1 ounce (due to its density being roughly 1 g/mL), the weight and volume measurements for water are nearly equivalent. However, this direct conversion does not apply to all substances. For example, substances less dense than water, like cooking oil, will have more fluid ounces in a pound than water, while denser substances, like honey, will have fewer fluid ounces in a pound.
Key Points
- The conversion between fluid ounces and pounds depends on the substance's density.
- For water, 1 pound is approximately equal to 16 fluid ounces due to its density being close to 1 g/mL.
- Substances less dense than water will have more fluid ounces in a pound, and denser substances will have fewer fluid ounces in a pound.
- Understanding the density of a substance is crucial for accurate conversions between volume and weight measurements.
- Recipes and chemical procedures often require precise conversions to ensure desired outcomes.
Understanding Density and Its Impact on Conversion

Density plays a critical role in the conversion between fluid ounces and pounds. It is defined as mass per unit volume and is typically expressed in units such as grams per cubic centimeter (g/cm³) or pounds per cubic foot (lb/ft³). The density of a substance determines how much of it will fit into a given volume. For instance, 1 fluid ounce of a substance with a density of 1 g/mL will weigh approximately 1 ounce, but if the substance has a different density, the weight of 1 fluid ounce will be different.
Calculating Fluid Ounces in a Pound for Different Substances
To calculate how many fluid ounces are in a pound of a particular substance, you need to know the density of that substance. The formula for this calculation is: fluid ounces = weight in ounces / (density in g/mL * 0.066667), where 0.066667 is a conversion factor from grams to ounces (since 1 ounce is approximately equal to 28.35 grams, and 1 fluid ounce of water weighs about 1 ounce). For substances with densities significantly different from water, this calculation will yield a different number of fluid ounces per pound.
| Substance | Density (g/mL) | Fluid Ounces per Pound |
|---|---|---|
| Water | 1 | 16 |
| Cooking Oil | 0.91 | 17.58 |
| Honey | 1.36 | 11.76 |

Practical Applications and Considerations
In practical applications, such as cooking, baking, or conducting chemical experiments, understanding the relationship between fluid ounces and pounds is vital. Recipes often call for ingredients by volume (fluid ounces) or by weight (pounds or ounces), and incorrect conversions can significantly affect the final product. For instance, in baking, where precise measurements are crucial, using the wrong conversion can lead to undesirable textures or flavors. Similarly, in chemistry, accurate measurements are essential for achieving the desired chemical reactions and avoiding potential hazards.
Moreover, the conversion between fluid ounces and pounds is also relevant in commercial and industrial settings, such as in the production of food, beverages, and pharmaceuticals. Here, precise control over ingredient quantities is not only a matter of quality but also of safety and regulatory compliance. Understanding and correctly applying the conversion principles can help in optimizing production processes, ensuring consistency in products, and minimizing waste.
Addressing Potential Objections or Limitations
One potential objection to the emphasis on precise conversions between fluid ounces and pounds is the argument that, for many practical purposes, approximate conversions may suffice. However, this perspective overlooks the critical importance of accuracy in many applications. Another limitation could be the challenge of determining the exact density of a substance, especially in mixed or complex materials. Addressing these limitations requires a nuanced understanding of the specific application, the properties of the substances involved, and the potential impacts of measurement errors.
How do I convert fluid ounces to pounds for substances other than water?
+To convert fluid ounces to pounds for substances other than water, you need to know the density of the substance. The formula involves dividing the weight in ounces by the product of the density in g/mL and a conversion factor. For precise calculations, refer to the substance's specific density and apply the formula accordingly.
Why is understanding the relationship between fluid ounces and pounds important?
+Understanding this relationship is crucial for achieving accurate results in recipes, chemical experiments, and industrial productions. Incorrect conversions can lead to undesirable outcomes, safety hazards, or non-compliance with regulations. Precise conversions ensure quality, safety, and efficiency in various applications.
How does the density of a substance affect the conversion between fluid ounces and pounds?
+The density of a substance directly affects the conversion because it determines how much of the substance fits into a given volume. Substances with densities less than that of water will have more fluid ounces in a pound, and substances with densities greater than that of water will have fewer fluid ounces in a pound. Knowing the density is essential for accurate conversions.
In conclusion, the conversion between fluid ounces and pounds is a nuanced topic that depends on the density of the substance being measured. Understanding this relationship is essential for various practical applications, ensuring accuracy, quality, and safety. By recognizing the importance of density in these conversions and applying the appropriate formulas, individuals can achieve precise measurements and desirable outcomes in their work, whether in cooking, chemistry, or industrial production.



