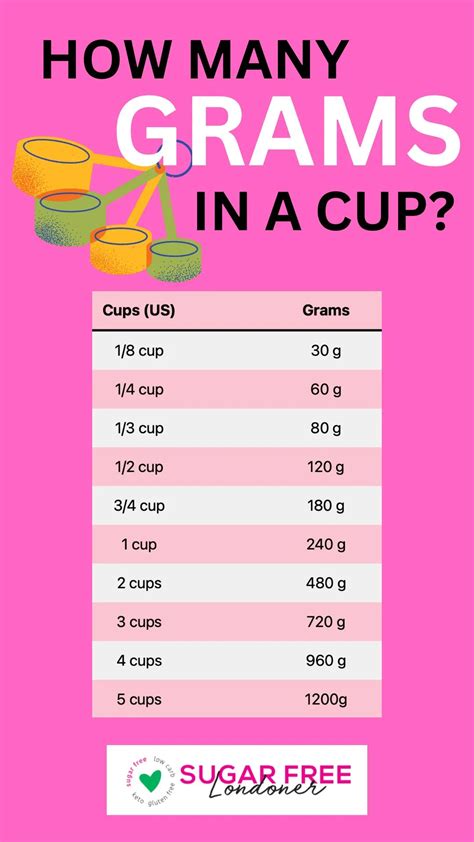
The relationship between grams and liters is fundamentally based on the density of the substance in question. When we're discussing water, the density is approximately 1 gram per milliliter (g/mL) at standard temperature and pressure (STP) conditions. This means that for every milliliter of water, there is approximately 1 gram of mass. Given that there are 1,000 milliliters in 1 liter, we can easily calculate the mass of 1 liter of water.
Calculating Grams in 1 Liter of Water
To find out how many grams are in 1 liter of water, we multiply the number of milliliters in a liter by the grams per milliliter. Thus, 1,000 mL * 1 g/mL = 1,000 grams. This calculation provides a straightforward answer to the question of how many grams are in 1 liter of water, demonstrating the direct relationship between volume and mass for water under standard conditions.
Density and Its Role
The key concept here is density, which is defined as mass per unit volume. For water, this density is close to 1 g/mL, making the calculation simple. However, it’s essential to note that this value can slightly vary with temperature and pressure changes, although for most practical purposes, 1 g/mL is a reliable approximation for the density of water.
| Volume of Water | Mass of Water |
|---|---|
| 1 Liter | 1,000 grams (or 1 kilogram) |

Key Points
- The density of water is approximately 1 gram per milliliter (g/mL) under standard conditions.
- There are 1,000 grams in 1 liter of water, based on the calculation 1,000 mL * 1 g/mL.
- Density is a critical factor in understanding the relationship between the volume and mass of substances, including water.
- Temperature and pressure can slightly affect the density of water, but for most purposes, 1 g/mL is a reliable value.
- Understanding the mass of water in different volumes is essential for various applications, including science, engineering, and everyday calculations.
Applications and Considerations
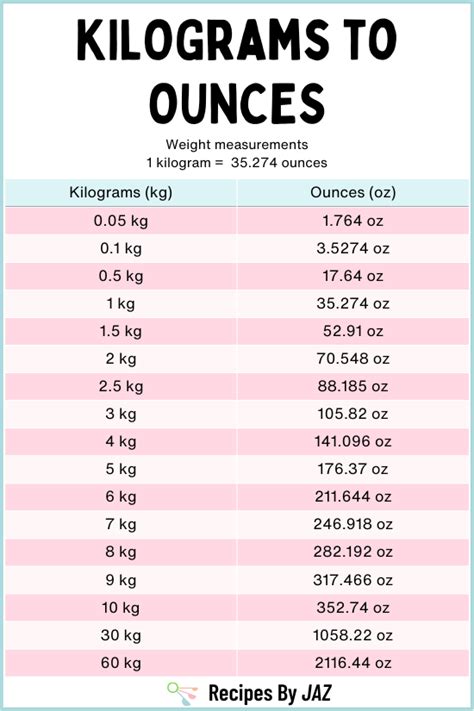
The knowledge of how many grams are in a liter of water has widespread applications. In cooking, understanding the mass of ingredients is crucial for recipe accuracy. In science and engineering, calculating the mass of water is essential for problems involving energy transfer, fluid dynamics, and structural integrity. Furthermore, in environmental studies, knowing the mass of water in different bodies (like lakes, rivers, and reservoirs) is vital for assessing water resources and managing ecosystems.
Practical Implications
In practical terms, recognizing that 1 liter of water weighs approximately 1 kilogram (since 1,000 grams = 1 kilogram) can be very useful. This equivalence is often used in everyday applications, from measuring ingredients for recipes to calculating the weight capacity of containers or vehicles. It also underscores the importance of precise measurement in both domestic and industrial contexts, where the mass of water can significantly impact outcomes.
Meta Description: Discover how many grams are in 1 liter of water and explore the significance of water's density in various applications, from cooking to engineering and environmental science.
What is the density of water under standard conditions?
+The density of water is approximately 1 gram per milliliter (g/mL) under standard temperature and pressure conditions.
How many grams are in 1 liter of water?
+There are 1,000 grams in 1 liter of water, as calculated by multiplying 1,000 mL (the volume of 1 liter) by 1 g/mL (the density of water).
Why is understanding the mass of water important?
+Understanding the mass of water is crucial for various applications, including cooking, science, engineering, and environmental studies, as it affects calculations involving energy, volume, and weight.